Globe Newswire02.06.19
BioSig Technologies Inc., a medical device company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the $4.6 billion electrophysiology (EP) marketplace, has appointed MaryAnn Edzards as senior director - account manager.
Edzards brings to the company over 10 years of experience in electrophysiology (EP), including six years at Biosense Webster, a Johnson & Johnson company. Most recently, she served as new technology education manager, a role, in which she was responsible for all internal and external marketing and training programs for two global product launches. Edzards facilitated training of over 300 field and in-house employees and delivered impactful training modules for complex technologies through virtual and live classroom environments. She brings to BioSig extensive experience in converting voice of customer feedback into commercially valuable solutions. Edzards is a holder of numerous Johnson & Johnson awards, including 2016 Standards of Leadership Award and 2017 Gold Encore Award from Commercial Marketing.
“A high-performing and motivated professional like MaryAnn is an invaluable addition to our commercial team. Her expertise in delivering highly impactful education and training of both in-house professionals and physicians will tremendously benefit our efforts during the vital First-in-Human patient data collection phase and subsequent market launch in 2019,” stated Mr. Kenneth Londoner, chairman and CEO of BioSig Technologies.
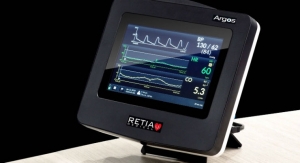
The company received U.S. Food and Drug Administration 510(k) clearance for its PURE EP System on Aug. 14, 2018. In late November, BioSig began installations of the first systems at Texas Cardiac Arrhythmia Institute in Austin, Texas; in early December, the company enrolled Mayo Clinic as the second center for the First-in-Human studies. BioSig signed a 10-year collaboration agreement with Mayo Clinic in March 2017 and announced a new research agreement focusing on development of additional advanced features and potential new applications of PURE EP System on Nov. 13, 2018.
“I’m excited to join the BioSig team as the Company commences first installations of PURE EP System in some of the leading medical centers of excellence. There is a pressing need for better technological solutions in the space of arrhythmia treatments, and I look forward to contributing my knowledge and expertise to help the Company bring its novel platform onto the market,” commented Edzards.
BioSig Technologies is a medical technology company developing a proprietary biomedical signal processing platform designed to improve the electrophysiology (EP) marketplace. Los Angeles, Calif.-based BioSig Technologies is preparing to commercialize its PURE EP System. The technology has been developed to address an unmet need in a large and growing market. The company’s first product, PURE EP System, is a cardiac signal acquisition and display system which is engineered to assist electrophysiologists in clinical decision-making during procedures to diagnose and treat patients with abnormal heart rates and rhythms. BioSig’s main goal is to deliver technology to improve upon catheter ablation treatments for the prevalent and potentially deadly arrhythmias, atrial fibrillation and ventricular tachycardia. BioSig has partnered with Minnetronix on technology development.
Edzards brings to the company over 10 years of experience in electrophysiology (EP), including six years at Biosense Webster, a Johnson & Johnson company. Most recently, she served as new technology education manager, a role, in which she was responsible for all internal and external marketing and training programs for two global product launches. Edzards facilitated training of over 300 field and in-house employees and delivered impactful training modules for complex technologies through virtual and live classroom environments. She brings to BioSig extensive experience in converting voice of customer feedback into commercially valuable solutions. Edzards is a holder of numerous Johnson & Johnson awards, including 2016 Standards of Leadership Award and 2017 Gold Encore Award from Commercial Marketing.
“A high-performing and motivated professional like MaryAnn is an invaluable addition to our commercial team. Her expertise in delivering highly impactful education and training of both in-house professionals and physicians will tremendously benefit our efforts during the vital First-in-Human patient data collection phase and subsequent market launch in 2019,” stated Mr. Kenneth Londoner, chairman and CEO of BioSig Technologies.
The company received U.S. Food and Drug Administration 510(k) clearance for its PURE EP System on Aug. 14, 2018. In late November, BioSig began installations of the first systems at Texas Cardiac Arrhythmia Institute in Austin, Texas; in early December, the company enrolled Mayo Clinic as the second center for the First-in-Human studies. BioSig signed a 10-year collaboration agreement with Mayo Clinic in March 2017 and announced a new research agreement focusing on development of additional advanced features and potential new applications of PURE EP System on Nov. 13, 2018.
“I’m excited to join the BioSig team as the Company commences first installations of PURE EP System in some of the leading medical centers of excellence. There is a pressing need for better technological solutions in the space of arrhythmia treatments, and I look forward to contributing my knowledge and expertise to help the Company bring its novel platform onto the market,” commented Edzards.
BioSig Technologies is a medical technology company developing a proprietary biomedical signal processing platform designed to improve the electrophysiology (EP) marketplace. Los Angeles, Calif.-based BioSig Technologies is preparing to commercialize its PURE EP System. The technology has been developed to address an unmet need in a large and growing market. The company’s first product, PURE EP System, is a cardiac signal acquisition and display system which is engineered to assist electrophysiologists in clinical decision-making during procedures to diagnose and treat patients with abnormal heart rates and rhythms. BioSig’s main goal is to deliver technology to improve upon catheter ablation treatments for the prevalent and potentially deadly arrhythmias, atrial fibrillation and ventricular tachycardia. BioSig has partnered with Minnetronix on technology development.