Montpellier PR01.03.19
ERADA Technology Alliance Ltd (ERADA), pioneers of innovative, rapid diagnostic solutions for early detection of infectious diseases, have announced the imminent launch of a world first diagnostic saliva test for malaria.
The saliva-based diagnostic tool, to be marketed by ERADA as a Saliva-based Malaria Asymptomatic and Asexual Rapid Test (SMAART) for subclinical infection, is set to transform malaria detection worldwide in the fight against one of the globe's most deadly diseases. Malaria, globally kills an estimated 435,000 each year, mostly children under the age of five, mainly in Sub-Saharan Africa.1
The SMAART detection tool is the invention of leading, U.S. based, researchers in the field of malaria diagnostics whose study was published worldwide January 2 in the international journal, Science Translational Medicine.
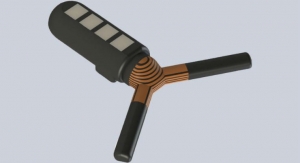
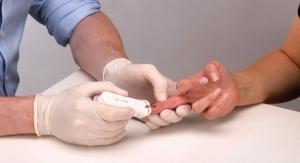
ERADA's innovative solution is easy-to-use, as it includes a simple device for standardized collection of saliva that can be implemented in the community by health care professionals, teachers and parents; contrasting with invasive blood tests, which must be administered by trained clinicians. Other drawbacks to blood tests include cultural 'blood taboos' existing in many countries whilst, furthermore, skin-prick tests are often stressful for children and parents.
Existing tests using blood may be invariably less reliable because subclinical infections with malaria-carrying parasites can be missed, leading some patients to come down with the disease, without knowing they have already been infected. ERADA's SMAART-1, easy-to-use saliva test, leads to early detection, treatment, and prevention of the disease as well as reducing further transmission of malaria.
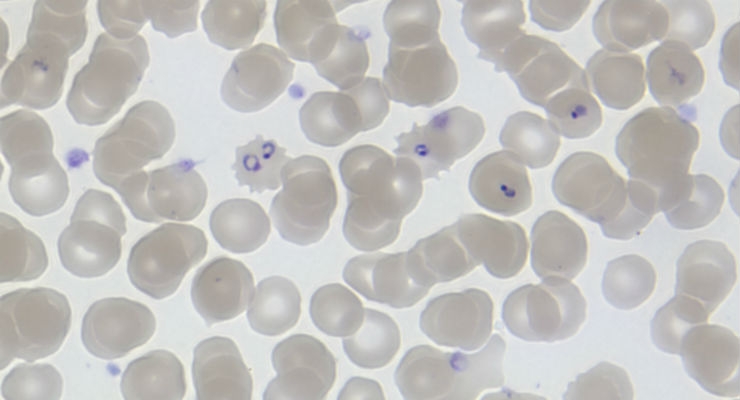
ERADA's SMAART saliva test detects a unique biomarker from female parasites circulating in an infected human who is asymptomatic, but is carrying the parasite and likely to come down with malaria within a week. Early, subclinical detection of malaria is crucial to malaria eradication because individuals who carry the parasite without exhibiting symptoms, known as carriers, are the reservoir that leads to infection of mosquitoes and transmission of the disease. Detecting the presence of the parasite before symptoms appear can save lives because malaria visible disease only erupts a couple of days after the mosquito bite.
The SMAART detection tool works by detecting a novel biomarker for Plasmodium falciparum parasites. In some areas of the world, the parasites have acquired a mutation and are therefore no longer detected by current blood-based tests. But ERADA's saliva test detects an essential protein the parasite needs for survival, which should avoid the problem of influence from the mutation and keep the test effective long-term.
"As someone who has suffered from malaria, I know first-hand that if the parasite had been detected early, I could have been treated and cured before the symptoms of the disease made me unwell," Dr. Benji Pretorius, ERADA's founder and Managing Director said.
Dr. Pretorius continued, "As a practicing clinician myself and following my personal experience of this debilitating disease, I was spurred on to work with my colleague Dr. Richard Schmidt in our small community, Musina, in South Africa, together with a global team of scientists." Dr. Pretorius continues, "Our vision is to bring to market ERADA's SMAART diagnostic tool as quickly as possible in the belief that it will go on to save literally millions of lives in the future."
The World Health Organization's recently published World Malaria Report 2018 reinforces the message that the world is currently behind 2020 milestones of the WHO Global Technical Strategy for Malaria 2016-2030.1 Reduction in malaria cases has stalled and of particular concern is the report's finding that, in 2017, there were an estimated 3.5 million more cases of malaria in the 10 highest burden African countries.1,3
"The introduction of SMAART is going to play a major part in achieving effective diagnostic testing and surveillance; as well as prevention and treatment of this disease, and therefore will be a major catalyst in meeting the WHO's 2030 target to reduce malaria incidence and mortality by 90 percent," Dr. Pretorius said.2
References
1 World malaria report. Geneva: World Health Organization; 2018: Available at https://www.who.int/malaria/publications/world-malaria-report-2018/en/
2 Global Technical Strategy for Malaria, 2016-2030. Geneva: World Health Organization; 2015: Available at https://www.who.int/malaria/publications/atoz/9789241564991/en/
3 https://www.who.int/news-room/detail/19-11-2018-who-and-partners-launch-new-country-led-response-to-put-stalled-malaria-control-efforts-back-on-track
The saliva-based diagnostic tool, to be marketed by ERADA as a Saliva-based Malaria Asymptomatic and Asexual Rapid Test (SMAART) for subclinical infection, is set to transform malaria detection worldwide in the fight against one of the globe's most deadly diseases. Malaria, globally kills an estimated 435,000 each year, mostly children under the age of five, mainly in Sub-Saharan Africa.1
The SMAART detection tool is the invention of leading, U.S. based, researchers in the field of malaria diagnostics whose study was published worldwide January 2 in the international journal, Science Translational Medicine.
ERADA's innovative solution is easy-to-use, as it includes a simple device for standardized collection of saliva that can be implemented in the community by health care professionals, teachers and parents; contrasting with invasive blood tests, which must be administered by trained clinicians. Other drawbacks to blood tests include cultural 'blood taboos' existing in many countries whilst, furthermore, skin-prick tests are often stressful for children and parents.
Existing tests using blood may be invariably less reliable because subclinical infections with malaria-carrying parasites can be missed, leading some patients to come down with the disease, without knowing they have already been infected. ERADA's SMAART-1, easy-to-use saliva test, leads to early detection, treatment, and prevention of the disease as well as reducing further transmission of malaria.
ERADA's SMAART saliva test detects a unique biomarker from female parasites circulating in an infected human who is asymptomatic, but is carrying the parasite and likely to come down with malaria within a week. Early, subclinical detection of malaria is crucial to malaria eradication because individuals who carry the parasite without exhibiting symptoms, known as carriers, are the reservoir that leads to infection of mosquitoes and transmission of the disease. Detecting the presence of the parasite before symptoms appear can save lives because malaria visible disease only erupts a couple of days after the mosquito bite.
The SMAART detection tool works by detecting a novel biomarker for Plasmodium falciparum parasites. In some areas of the world, the parasites have acquired a mutation and are therefore no longer detected by current blood-based tests. But ERADA's saliva test detects an essential protein the parasite needs for survival, which should avoid the problem of influence from the mutation and keep the test effective long-term.
"As someone who has suffered from malaria, I know first-hand that if the parasite had been detected early, I could have been treated and cured before the symptoms of the disease made me unwell," Dr. Benji Pretorius, ERADA's founder and Managing Director said.
Dr. Pretorius continued, "As a practicing clinician myself and following my personal experience of this debilitating disease, I was spurred on to work with my colleague Dr. Richard Schmidt in our small community, Musina, in South Africa, together with a global team of scientists." Dr. Pretorius continues, "Our vision is to bring to market ERADA's SMAART diagnostic tool as quickly as possible in the belief that it will go on to save literally millions of lives in the future."
The World Health Organization's recently published World Malaria Report 2018 reinforces the message that the world is currently behind 2020 milestones of the WHO Global Technical Strategy for Malaria 2016-2030.1 Reduction in malaria cases has stalled and of particular concern is the report's finding that, in 2017, there were an estimated 3.5 million more cases of malaria in the 10 highest burden African countries.1,3
"The introduction of SMAART is going to play a major part in achieving effective diagnostic testing and surveillance; as well as prevention and treatment of this disease, and therefore will be a major catalyst in meeting the WHO's 2030 target to reduce malaria incidence and mortality by 90 percent," Dr. Pretorius said.2
References
1 World malaria report. Geneva: World Health Organization; 2018: Available at https://www.who.int/malaria/publications/world-malaria-report-2018/en/
2 Global Technical Strategy for Malaria, 2016-2030. Geneva: World Health Organization; 2015: Available at https://www.who.int/malaria/publications/atoz/9789241564991/en/
3 https://www.who.int/news-room/detail/19-11-2018-who-and-partners-launch-new-country-led-response-to-put-stalled-malaria-control-efforts-back-on-track