Business Wire12.18.18
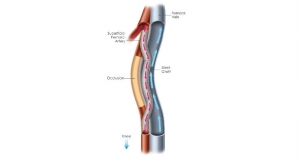
Surmodics Inc., a provider of medical device and in-vitro diagnostic technologies, has shared data from the PREVEIL early feasibility study (EFS) of the company’s SurVeil drug-coated balloon (DCB). PREVEIL is a prospective, U.S., multi-center, single-arm trial designed to assess the safety and feasibility of the SurVeil DCB in the treatment of subjects with symptomatic peripheral artery disease (PAD) due to de novo lesions of the femoral and popliteal arteries.
Twelve-month data from the study show that acute success measures of safety were achieved in 100 percent of subjects. No subjects required re-intervention of either the target lesion or the target vessel at 12 months (100 percent freedom from clinically driven target lesion revascularization and target vessel revascularization (CD-TLR or CD-TVR)). The results also demonstrate continued significant improvement in Rutherford classification, resting ankle brachial index (ABI), and walking impairment questionnaire (WIQ) including walking distance, walking speed and stair-climbing scores at 12 months. As was presented with the six-month results, median paclitaxel plasma concentration peaked immediately post-procedure (Cmax 1.07 ng/mL) and was undetectable at 30 days. Secondary technical, device, and procedure success criteria were achieved. The SurVeil DCB is not yet approved for sale in the United States.
“The ongoing positive results from this study demonstrate that the SurVeil DCB has the potential to be a next-generation DCB with improved efficacy of drug transfer,” said Kenneth Rosenfield, M.D., section head, Vascular Medicine and Intervention at Massachusetts General Hospital, and U.S. co-principal investigator for the TRANSCEND trial. “These 12-month data continue to support the functionality and safety of the device.”
Data presented include 12-month results from 13 patients (Rutherford class 2 to 4) at three clinical sites who were treated with the SurVeil DCB. Average lesion length was 56 mm. Clinical assessments for the study include primary patency and late lumen loss through six months, plasma paclitaxel levels, and changes in Rutherford classification, ABI/TBI, 6-minute walk test, and WIQ at 1, 6, 12, 24 and 36 months. Key secondary safety endpoints included freedom from major vascular complications, evidence of paclitaxel toxicity, or thrombolysis in myocardial infarction (TIMI).
“Our goal all along with the SurVeil DCB has been to advance the technology to improve drug transfer and distribution effect on the arterial wall offering the opportunity to use a lower drug dose,” said Gary Maharaj, president and CEO of Surmodics. “We are pleased with the ongoing results from the EFS and look forward to the opportunity to continue to demonstrate the potential for this technology with outcomes from our pivotal TRANSCEND clinical trial that is currently underway.”
In July 2017, Surmodics received an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to initiate a pivotal clinical trial of the SurVeil DCB, and the first patient was enrolled in October 2017. The randomized trial, TRANSCEND, is designed to evaluate the SurVeil DCB for treatment for PAD in the upper leg compared to the Medtronic IN.PACT Admiral DCB.
The design of the SurVeil DCB reflects Surmodics’ long-standing industry leadership in the development of surface technology for vascular medical devices. The device includes a proprietary drug-excipient formulation for the balloon coating and is manufactured using a proprietary process to improve coating uniformity. Pre-clinical data have shown a three to five times higher target tissue drug concentration, a more evenly distributed and durable drug effect, and lower incidence of downstream drug concentrations compared to control DCBs.1
Surmodics is the global leader in surface modification technologies for intravascular medical devices and a provider of chemical components for in-vitro diagnostic (IVD) immunoassay tests and microarrays. Surmodics is pursuing highly differentiated whole-product solutions that are designed to address unmet clinical needs for its medical device customers and engineered to the most demanding requirements. This key growth strategy leverages the combination of the company’s expertise in proprietary surface technologies, along with enhanced device design, development and manufacturing capabilities. The company mission remains to improve the detection and treatment of disease. Surmodics is headquartered in Eden Prairie, Minn.
Reference
1. Surmodics data on file
Twelve-month data from the study show that acute success measures of safety were achieved in 100 percent of subjects. No subjects required re-intervention of either the target lesion or the target vessel at 12 months (100 percent freedom from clinically driven target lesion revascularization and target vessel revascularization (CD-TLR or CD-TVR)). The results also demonstrate continued significant improvement in Rutherford classification, resting ankle brachial index (ABI), and walking impairment questionnaire (WIQ) including walking distance, walking speed and stair-climbing scores at 12 months. As was presented with the six-month results, median paclitaxel plasma concentration peaked immediately post-procedure (Cmax 1.07 ng/mL) and was undetectable at 30 days. Secondary technical, device, and procedure success criteria were achieved. The SurVeil DCB is not yet approved for sale in the United States.
“The ongoing positive results from this study demonstrate that the SurVeil DCB has the potential to be a next-generation DCB with improved efficacy of drug transfer,” said Kenneth Rosenfield, M.D., section head, Vascular Medicine and Intervention at Massachusetts General Hospital, and U.S. co-principal investigator for the TRANSCEND trial. “These 12-month data continue to support the functionality and safety of the device.”
Data presented include 12-month results from 13 patients (Rutherford class 2 to 4) at three clinical sites who were treated with the SurVeil DCB. Average lesion length was 56 mm. Clinical assessments for the study include primary patency and late lumen loss through six months, plasma paclitaxel levels, and changes in Rutherford classification, ABI/TBI, 6-minute walk test, and WIQ at 1, 6, 12, 24 and 36 months. Key secondary safety endpoints included freedom from major vascular complications, evidence of paclitaxel toxicity, or thrombolysis in myocardial infarction (TIMI).
“Our goal all along with the SurVeil DCB has been to advance the technology to improve drug transfer and distribution effect on the arterial wall offering the opportunity to use a lower drug dose,” said Gary Maharaj, president and CEO of Surmodics. “We are pleased with the ongoing results from the EFS and look forward to the opportunity to continue to demonstrate the potential for this technology with outcomes from our pivotal TRANSCEND clinical trial that is currently underway.”
In July 2017, Surmodics received an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to initiate a pivotal clinical trial of the SurVeil DCB, and the first patient was enrolled in October 2017. The randomized trial, TRANSCEND, is designed to evaluate the SurVeil DCB for treatment for PAD in the upper leg compared to the Medtronic IN.PACT Admiral DCB.
The design of the SurVeil DCB reflects Surmodics’ long-standing industry leadership in the development of surface technology for vascular medical devices. The device includes a proprietary drug-excipient formulation for the balloon coating and is manufactured using a proprietary process to improve coating uniformity. Pre-clinical data have shown a three to five times higher target tissue drug concentration, a more evenly distributed and durable drug effect, and lower incidence of downstream drug concentrations compared to control DCBs.1
Surmodics is the global leader in surface modification technologies for intravascular medical devices and a provider of chemical components for in-vitro diagnostic (IVD) immunoassay tests and microarrays. Surmodics is pursuing highly differentiated whole-product solutions that are designed to address unmet clinical needs for its medical device customers and engineered to the most demanding requirements. This key growth strategy leverages the combination of the company’s expertise in proprietary surface technologies, along with enhanced device design, development and manufacturing capabilities. The company mission remains to improve the detection and treatment of disease. Surmodics is headquartered in Eden Prairie, Minn.
Reference
1. Surmodics data on file