PR Newswire12.04.18
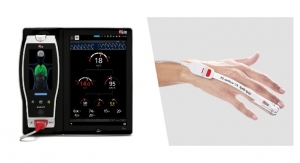
Notal Vision, Ltd., a privately-held ophthalmic diagnostic services company, focused on advancing eye care by extending ophthalmic disease management from the clinic to the home, has announced the U.S. Food and Drug Administration (FDA) granted the Notal Vision Home-based Optical Coherence Tomography (OCT) System with "Breakthrough Device" designation for the agreed upon indications for use. This designation indicates that FDA intends to provide interactive and timely communication with the sponsor during device development and throughout the review process for various types of premarket submissions.
FDA's Breakthrough Devices Program was established in late 2016 to help patients gain timely access to breakthrough technologies that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases, for which no approved or cleared treatment exists or that offer significant advantages over existing or cleared alternatives.
The indication for use statement for Notal's Home OCT System conveys that it is "an Artificial Intelligence (AI)-based Home Use device indicated for automated identification of intra- and/or subretinal fluid in the central 10 degrees of eyes diagnosed with exudative age-related macular degeneration (eAMD). The Notal Home OCT device is intended for testing at home between regularly scheduled clinic assessments and not intended to replace standard-of-care regularly scheduled examinations and clinical testing by an ophthalmic retinal specialist."
Dr. Susan Orr, chief medical officer at Notal Vision and incoming CEO as of January 1, 2019 stated, "We are very pleased with the FDA's acceptance of our request for Breakthrough Designation. We are eager to work closely with them to bring home-OCT testing to patients with exudative AMD, leveraging leading-edge technology to the benefit of patients, caregivers, and eye care providers alike."
The Notal Home OCT is a patient-friendly light-weight device designed for technician-free operation by eAMD patients from the comfort of their home. Once a patient completes the test, a proprietary machine-learning algorithm, the Notal OCT Analyzer (NOA™), performs an automated analysis. If retina fluid is detected, a report is generated by NOA™ which is then conveyed to the treating physician by the Notal Vision Diagnostic Clinic. The Notal Vision Home OCT will complement current disease monitoring strategies by providing retinal specialists with immediate notification if recurrent disease activity is detected, thereby reducing the time from fluid onset to next treatment.
"The FDA's Breakthrough Devices Program is designed to help expedite patient access to novel technologies through intensive interaction and guidance," said Quinton Oswald, CEO of Notal Vision. "This designation validates and reaffirms our belief that home-based OCT addresses a high unmet need for clinicians and their patients. We are excited about the FDA's recognition of the potential clinical benefit to the over one million Americans living with exudative AMD."
Notal Vision anticipates bringing the Notal Vision Home OCT System to the market in 2020.
FDA's Breakthrough Devices Program was established in late 2016 to help patients gain timely access to breakthrough technologies that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases, for which no approved or cleared treatment exists or that offer significant advantages over existing or cleared alternatives.
The indication for use statement for Notal's Home OCT System conveys that it is "an Artificial Intelligence (AI)-based Home Use device indicated for automated identification of intra- and/or subretinal fluid in the central 10 degrees of eyes diagnosed with exudative age-related macular degeneration (eAMD). The Notal Home OCT device is intended for testing at home between regularly scheduled clinic assessments and not intended to replace standard-of-care regularly scheduled examinations and clinical testing by an ophthalmic retinal specialist."
Dr. Susan Orr, chief medical officer at Notal Vision and incoming CEO as of January 1, 2019 stated, "We are very pleased with the FDA's acceptance of our request for Breakthrough Designation. We are eager to work closely with them to bring home-OCT testing to patients with exudative AMD, leveraging leading-edge technology to the benefit of patients, caregivers, and eye care providers alike."
The Notal Home OCT is a patient-friendly light-weight device designed for technician-free operation by eAMD patients from the comfort of their home. Once a patient completes the test, a proprietary machine-learning algorithm, the Notal OCT Analyzer (NOA™), performs an automated analysis. If retina fluid is detected, a report is generated by NOA™ which is then conveyed to the treating physician by the Notal Vision Diagnostic Clinic. The Notal Vision Home OCT will complement current disease monitoring strategies by providing retinal specialists with immediate notification if recurrent disease activity is detected, thereby reducing the time from fluid onset to next treatment.
"The FDA's Breakthrough Devices Program is designed to help expedite patient access to novel technologies through intensive interaction and guidance," said Quinton Oswald, CEO of Notal Vision. "This designation validates and reaffirms our belief that home-based OCT addresses a high unmet need for clinicians and their patients. We are excited about the FDA's recognition of the potential clinical benefit to the over one million Americans living with exudative AMD."
Notal Vision anticipates bringing the Notal Vision Home OCT System to the market in 2020.