Messe Düsseldorf11.12.18
During the first few years of the HIV epidemic, a person who developed AIDS could expect to live for less than a year.1 In San Francisco, Calif., between 1981 and 1985—
the year the first blood test for HIV was developed2—
median survival after AIDS was diagnosed was just 10.1-12.6 months.1 Today, the life expectancy for a person who is diagnosed and starts antiretroviral treatment (ART) rapidly after HIV infection probably approaches that of the general population.3
The World Health Organization (WHO) set a global target that 90 percent of all people living with HIV know their status, 90 percent of those diagnosed as HIV positive start ART, and 90 percent of all people receiving ART show durable viral suppression.4 In 2017, these proportions were 75 percent, 79 percent, and 81 percent respectively.5 Moving closer to the WHO targets depends on focusing on groups and areas with a high prevalence and improving access to high-quality tests to diagnose and monitor HIV.4 Currently, however, many people with HIV are diagnosed late despite the widespread availability of accurate tests.6, 7, 8, 9
Broadly, late diagnosis means that a person is diagnosed when his or her CD4+ T-cell count is below 350 cells/mm.3 A person who is diagnosed when his or her CD4+ T-cell count is below 200 cells/mm3 has ‘advanced HIV disease’ (AHD). Patients at these counts may be asymptomatic or present with an AIDS-defining event (illness), such as Kaposi’s sarcoma, Pneumocystis jiroveci pneumonia or cytomegalovirus retinitis.
People who start ART when their CD4+ count is below 350 cells/mm3 show a worse prognosis and have greater healthcare costs than those with higher counts.8 During the Insight Start study, for example, 4685 HIV-positive adults with CD4+ counts of more than 500 cells/mm3 started ART immediately or waited until their count fell to 350 cells/mm3 or they developed AIDS or another condition needing ART. The primary end point was any serious AIDS-related event, serious non–AIDS-related event, or death from any cause.10
During the average follow up of three years, the primary end point was 57 percent less common in those who started ART immediately. Serious AIDS-related events and serious non–AIDS-related events were 72 percent and 39 percent less likely respectively in those who started ART immediately.10
A study from England and Wales reported that people who were diagnosed late were 3.5 times more likely to die, usually from AIDS-related events. Mortality was 10 times higher during the year after a late diagnosis compared to those who were diagnosed rapidly.3 People diagnosed late are also typically unaware that they are HIV positive for approximately three to five years6 and, as a result, are more likely to transmit HIV than those diagnosed promptly.8
Despite the well-established benefits of early ART, the proportion of people diagnosed late in the United Kingdom remained approximately 40 percent between 2012 and 2017, when 43 percent of diagnoses were late. Moreover, 5 percent had an AIDS-defining disease at diagnosis.6
A Israeli study reported that of 356 patients with HIV, 33 percent were diagnosed late and 16 percent had AHD.7 In 20,965 patients from Holland, 53 percent were diagnosed late and 35 percent had AHD. However, the proportion of people with late presentation decreased from 62 percent in 1996 to 42 percent in 2013. The proportion with AHD decreased from 46 percent to 26 percent during this time.8
Overall, the European Centre for Disease Prevention and Control estimates that, depending on the country, up to 43 percent of people living with HIV in Europe remain undiagnosed. On average, 47 percent of HIV cases are diagnosed late and 28 percent of people have AHD. The proportion of people with HIV diagnosed late exceeds 50 percent in nine European countries, including Italy, Greece and Sweden.9
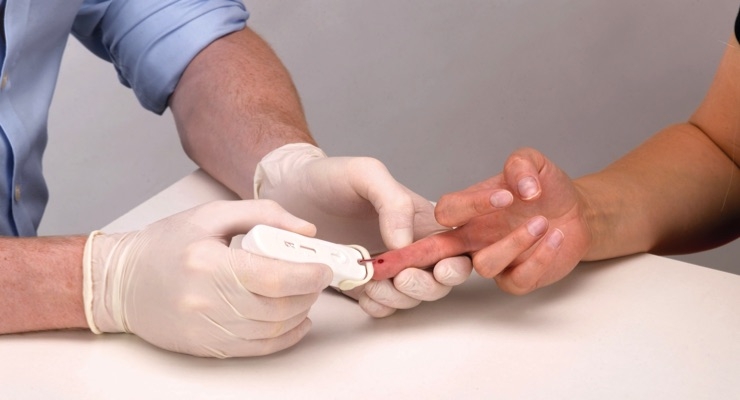
The introduction of the Simplitude Pro HIV (1&2) rapid diagnosis test at Medica 2018 in Düsseldorf, Germamy, addresses this issue, meeting the need for a reliable point-of-care diagnostic test (the overall sensitivity and specificity is 99.6 percent) that is simple to use and offers patients and healthcare workers the confidence to start ART and take steps to prevent transmission.
The Simplitude Pro HIV (1&2) is the result of a strategic partnership between Owen Mumford, a leading player in the medical device industry and Atomo Diagnostics, a product design and manufacturing company based in Australia that specializes in the development of integrated diagnostic test devices.
The integrated device with built-in lancet and blood collection unit enables the healthcare professional to collect and transfer the correct amount of blood and conduct the steps necessary to perform the test accurately. This addresses several of the key challenges identified with rapid diagnostic tests (RDTs) such as under collection of blood and complicated instructions which research has been shown to lead to user error.
“Earlier diagnosis enables people with HIV to start ART sooner, which increases their chances of living a long and healthy life and reduces the risk of transmission,” said Tania MacKenzie, senior product manager for Diagnostics at Owen Mumford Ltd., developer of the Simplitude Pro HIV test. “We believe that the convenience and prompt results offered by point-of-care tests, such as Simplitude Pro HIV (1&2), in community-based settings worldwide will help reduce the number of patients diagnosed late and so further improve outcomes for people living with HIV.”
Owen Mumford is a global industry leader in medical device design and manufacturing. The company develops medical products under its own Owen Mumford brands as well as custom device solutions for pharmaceutical and diagnostic companies.
References
1. Lemp GF, Payne SF, Neal D et al Survival trends for patients with AIDS JAMA 1990;263:402-406
2. Greene WC A history of AIDS: Looking back to see ahead European Journal of Immunology 2007;37:S94-S102
3. Croxford S, Kitching A, Desai S et al Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: An analysis of a national observational cohort The Lancet Public Health 2017;2:e35-e46
4. Habiyambere V, Dongmo Nguimfack B, Vojnov L et al Forecasting the global demand for HIV monitoring and diagnostic tests: A 2016-2021 analysis PLOS ONE 2018;13:e0201341
5. UNAIDS 2017 global HIV statistics Available at www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
6. Public Health England Trends in new HIV diagnoses and people receiving HIV-related care in the United Kingdom: data to the end of December 2017 Available at assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/738222/hpr3218_hiv17_v2.pdf
7. Levy I, Maor Y, Mahroum N et al Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: A retrospective cohort study BMJ Open 2016;6:e012721. DOI:10.1136/bmjopen-2016-012721
8. Op de Coul ELM, van Sighem A, Brinkman K et al Factors associated with presenting late or with advanced HIV disease in the Netherlands, 1996–2014: results from a national observational cohort BMJ Open 2016;6:e009688. 10.1136/bmjopen-2015-009688
9. European Centre for Disease Prevention and Control HIV testing Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 progress report Available at ecdc.europa.eu/sites/portal/files/documents/HIV%20testing.pdf
10. The INSIGHT START Study Group Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection New England Journal of Medicine 2015;373:795-807
The World Health Organization (WHO) set a global target that 90 percent of all people living with HIV know their status, 90 percent of those diagnosed as HIV positive start ART, and 90 percent of all people receiving ART show durable viral suppression.4 In 2017, these proportions were 75 percent, 79 percent, and 81 percent respectively.5 Moving closer to the WHO targets depends on focusing on groups and areas with a high prevalence and improving access to high-quality tests to diagnose and monitor HIV.4 Currently, however, many people with HIV are diagnosed late despite the widespread availability of accurate tests.6, 7, 8, 9
Broadly, late diagnosis means that a person is diagnosed when his or her CD4+ T-cell count is below 350 cells/mm.3 A person who is diagnosed when his or her CD4+ T-cell count is below 200 cells/mm3 has ‘advanced HIV disease’ (AHD). Patients at these counts may be asymptomatic or present with an AIDS-defining event (illness), such as Kaposi’s sarcoma, Pneumocystis jiroveci pneumonia or cytomegalovirus retinitis.
People who start ART when their CD4+ count is below 350 cells/mm3 show a worse prognosis and have greater healthcare costs than those with higher counts.8 During the Insight Start study, for example, 4685 HIV-positive adults with CD4+ counts of more than 500 cells/mm3 started ART immediately or waited until their count fell to 350 cells/mm3 or they developed AIDS or another condition needing ART. The primary end point was any serious AIDS-related event, serious non–AIDS-related event, or death from any cause.10
During the average follow up of three years, the primary end point was 57 percent less common in those who started ART immediately. Serious AIDS-related events and serious non–AIDS-related events were 72 percent and 39 percent less likely respectively in those who started ART immediately.10
A study from England and Wales reported that people who were diagnosed late were 3.5 times more likely to die, usually from AIDS-related events. Mortality was 10 times higher during the year after a late diagnosis compared to those who were diagnosed rapidly.3 People diagnosed late are also typically unaware that they are HIV positive for approximately three to five years6 and, as a result, are more likely to transmit HIV than those diagnosed promptly.8
Despite the well-established benefits of early ART, the proportion of people diagnosed late in the United Kingdom remained approximately 40 percent between 2012 and 2017, when 43 percent of diagnoses were late. Moreover, 5 percent had an AIDS-defining disease at diagnosis.6
A Israeli study reported that of 356 patients with HIV, 33 percent were diagnosed late and 16 percent had AHD.7 In 20,965 patients from Holland, 53 percent were diagnosed late and 35 percent had AHD. However, the proportion of people with late presentation decreased from 62 percent in 1996 to 42 percent in 2013. The proportion with AHD decreased from 46 percent to 26 percent during this time.8
Overall, the European Centre for Disease Prevention and Control estimates that, depending on the country, up to 43 percent of people living with HIV in Europe remain undiagnosed. On average, 47 percent of HIV cases are diagnosed late and 28 percent of people have AHD. The proportion of people with HIV diagnosed late exceeds 50 percent in nine European countries, including Italy, Greece and Sweden.9
The introduction of the Simplitude Pro HIV (1&2) rapid diagnosis test at Medica 2018 in Düsseldorf, Germamy, addresses this issue, meeting the need for a reliable point-of-care diagnostic test (the overall sensitivity and specificity is 99.6 percent) that is simple to use and offers patients and healthcare workers the confidence to start ART and take steps to prevent transmission.
The Simplitude Pro HIV (1&2) is the result of a strategic partnership between Owen Mumford, a leading player in the medical device industry and Atomo Diagnostics, a product design and manufacturing company based in Australia that specializes in the development of integrated diagnostic test devices.
The integrated device with built-in lancet and blood collection unit enables the healthcare professional to collect and transfer the correct amount of blood and conduct the steps necessary to perform the test accurately. This addresses several of the key challenges identified with rapid diagnostic tests (RDTs) such as under collection of blood and complicated instructions which research has been shown to lead to user error.
“Earlier diagnosis enables people with HIV to start ART sooner, which increases their chances of living a long and healthy life and reduces the risk of transmission,” said Tania MacKenzie, senior product manager for Diagnostics at Owen Mumford Ltd., developer of the Simplitude Pro HIV test. “We believe that the convenience and prompt results offered by point-of-care tests, such as Simplitude Pro HIV (1&2), in community-based settings worldwide will help reduce the number of patients diagnosed late and so further improve outcomes for people living with HIV.”
Owen Mumford is a global industry leader in medical device design and manufacturing. The company develops medical products under its own Owen Mumford brands as well as custom device solutions for pharmaceutical and diagnostic companies.
References
1. Lemp GF, Payne SF, Neal D et al Survival trends for patients with AIDS JAMA 1990;263:402-406
2. Greene WC A history of AIDS: Looking back to see ahead European Journal of Immunology 2007;37:S94-S102
3. Croxford S, Kitching A, Desai S et al Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: An analysis of a national observational cohort The Lancet Public Health 2017;2:e35-e46
4. Habiyambere V, Dongmo Nguimfack B, Vojnov L et al Forecasting the global demand for HIV monitoring and diagnostic tests: A 2016-2021 analysis PLOS ONE 2018;13:e0201341
5. UNAIDS 2017 global HIV statistics Available at www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
6. Public Health England Trends in new HIV diagnoses and people receiving HIV-related care in the United Kingdom: data to the end of December 2017 Available at assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/738222/hpr3218_hiv17_v2.pdf
7. Levy I, Maor Y, Mahroum N et al Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: A retrospective cohort study BMJ Open 2016;6:e012721. DOI:10.1136/bmjopen-2016-012721
8. Op de Coul ELM, van Sighem A, Brinkman K et al Factors associated with presenting late or with advanced HIV disease in the Netherlands, 1996–2014: results from a national observational cohort BMJ Open 2016;6:e009688. 10.1136/bmjopen-2015-009688
9. European Centre for Disease Prevention and Control HIV testing Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 progress report Available at ecdc.europa.eu/sites/portal/files/documents/HIV%20testing.pdf
10. The INSIGHT START Study Group Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection New England Journal of Medicine 2015;373:795-807