V-Wave Ltd.11.06.18
V-Wave Ltd. has enrolled the first patients in its global, 500-patient pivotal study of its proprietary, minimally invasive implanted interatrial shunt device for treating patients with NHYA Class III and ambulatory Class IV symptomatic heart failure (HF). This randomized, controlled, double-blinded multicenter clinical trial—the RELIEVE-HF study
—
will evaluate the safety and effectiveness of V-Wave's device therapy in severe HF patients with either preserved or reduced ejection fraction. The study is funded by V-Wave's $70 million early 2018 C-Round financing.
"Elevations in left atrial pressure (LAP) cause fluid to back-up in the lungs resulting in difficulty breathing; uncontrolled elevations in LAP are the most common cause (>90 percent) of acute hospitalizations for HF," noted Dr. Gregg W. Stone, professor of Medicine at Columbia University and director of Cardiovascular Research and Education at the Center for Interventional Vascular Therapy at New York-Presbyterian Hospital/Columbia University Medical Center. "The shunt continuously regulates LAP without patient or physician intervention by diverting a small portion of blood flow from the left to the right atrium, preventing uncontrolled LAP elevations. The RELIEVE HF trial will study the safety and effectiveness of the V-Wave shunt in patients with heart failure who remain highly symptomatic despite best medical treatments."
The first two patients in the RELIEVE-HF study were successfully implanted and discharged home the following morning from The Ohio State University Wexner Medical Center. Dr. Garrie Haas, director of the Heart Failure and Transplant Program is principal investigator and Drs. Scott Lilly (interventional cardiologist) and Rami Kahwash (heart failure cardiologist) are responsible for implantation and patient management, respectively.
Approximately 50 major North American hospitals are expected to participate in the pivotal study with up to another 25 centers in the European Union and Israel. Further information on the study is available at https://clinicaltrials.gov/ct2/show/NCT03499236.
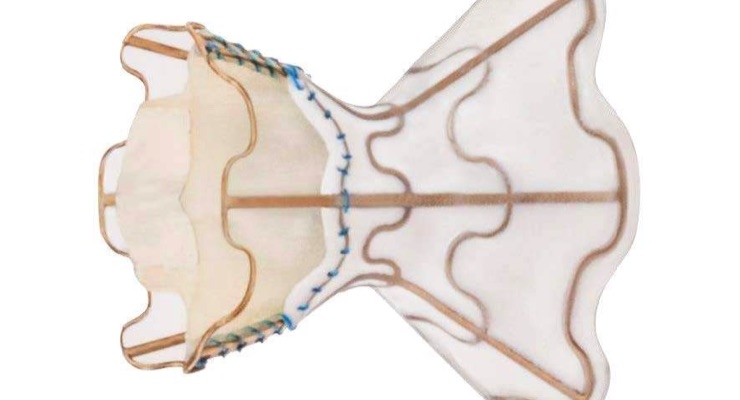
V-Wave CEO Dr. Neal Eigler described that "The interatrial shunt provides a novel therapeutic approach for patients with chronic HF. The shunt is implanted through a catheter inserted in a vein in the leg. Prior clinical experience demonstrated device and implantation procedure safety, and statistically significant improvements in symptoms, quality of life measurements, and exercise capacity were observed compared to a matched population receiving optimal care. We are fortunate to have our study designed by the world's leading HF clinical trialists: Drs. William Abraham from the Ohio State University, Gregg Stone, from Columbia University, JoAnn Lindenfeld of Vanderbilt University, Stefan D. Anker from Charité Berlin, Germany, and Josep Rodés-Cabau, M.D., Laval University, Quebec. These physicians have planned and executed some of the most influential and successful HF and structural heart disease device trials."
The study protocol allows inclusion of a broad pool of patients who are expected to have the best chance of benefitting from the treatment. The primary effectiveness outcome measure includes a hierarchical composite of mortality, heart transplant or ventricular assist device implantation, HF hospitalizations, and change in six-minute walk test distance.
With more than 26 million people suffering from chronic heart failure globally (including 6 million people in the United States), heart failure is the leading cause of hospitalizations in many countries; in the United States, it is Medicare's largest expense for acute hospitalization. Heart failure patients experience repeated hospitalizations, a poor quality of life, and a greatly reduced life expectancy.
V-Wave is a privately held medical device company with offices in Israel and the United States. The V-Wave Interatrial Shunt System is under clinical investigation and is not available for sale in the United States or other countries.
"Elevations in left atrial pressure (LAP) cause fluid to back-up in the lungs resulting in difficulty breathing; uncontrolled elevations in LAP are the most common cause (>90 percent) of acute hospitalizations for HF," noted Dr. Gregg W. Stone, professor of Medicine at Columbia University and director of Cardiovascular Research and Education at the Center for Interventional Vascular Therapy at New York-Presbyterian Hospital/Columbia University Medical Center. "The shunt continuously regulates LAP without patient or physician intervention by diverting a small portion of blood flow from the left to the right atrium, preventing uncontrolled LAP elevations. The RELIEVE HF trial will study the safety and effectiveness of the V-Wave shunt in patients with heart failure who remain highly symptomatic despite best medical treatments."
The first two patients in the RELIEVE-HF study were successfully implanted and discharged home the following morning from The Ohio State University Wexner Medical Center. Dr. Garrie Haas, director of the Heart Failure and Transplant Program is principal investigator and Drs. Scott Lilly (interventional cardiologist) and Rami Kahwash (heart failure cardiologist) are responsible for implantation and patient management, respectively.
Approximately 50 major North American hospitals are expected to participate in the pivotal study with up to another 25 centers in the European Union and Israel. Further information on the study is available at https://clinicaltrials.gov/ct2/show/NCT03499236.
V-Wave CEO Dr. Neal Eigler described that "The interatrial shunt provides a novel therapeutic approach for patients with chronic HF. The shunt is implanted through a catheter inserted in a vein in the leg. Prior clinical experience demonstrated device and implantation procedure safety, and statistically significant improvements in symptoms, quality of life measurements, and exercise capacity were observed compared to a matched population receiving optimal care. We are fortunate to have our study designed by the world's leading HF clinical trialists: Drs. William Abraham from the Ohio State University, Gregg Stone, from Columbia University, JoAnn Lindenfeld of Vanderbilt University, Stefan D. Anker from Charité Berlin, Germany, and Josep Rodés-Cabau, M.D., Laval University, Quebec. These physicians have planned and executed some of the most influential and successful HF and structural heart disease device trials."
The study protocol allows inclusion of a broad pool of patients who are expected to have the best chance of benefitting from the treatment. The primary effectiveness outcome measure includes a hierarchical composite of mortality, heart transplant or ventricular assist device implantation, HF hospitalizations, and change in six-minute walk test distance.
With more than 26 million people suffering from chronic heart failure globally (including 6 million people in the United States), heart failure is the leading cause of hospitalizations in many countries; in the United States, it is Medicare's largest expense for acute hospitalization. Heart failure patients experience repeated hospitalizations, a poor quality of life, and a greatly reduced life expectancy.
V-Wave is a privately held medical device company with offices in Israel and the United States. The V-Wave Interatrial Shunt System is under clinical investigation and is not available for sale in the United States or other countries.