Business Wire09.07.18
Thermedical said the U.S. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for the Early Feasibility Study of the Durablate ablation catheter. The single-arm, observational study is designed to evaluate the safety and effectiveness of the Durablate catheter to treat ventricular tachycardia (VT), a leading cause of sudden cardiac death in the United States.
The target population consists of patients who have already been treated with medicine, have an implantable cardioverter defibrillator (ICD), and who have had a conventional ablation procedure to treat VT, yet all their treatments have failed. The study is scheduled to begin in 2018 at Mayo Clinic under the direction of Douglas L. Packer, M.D., professor of medicine, and at Loyola University of Chicago Stritch School of Medicine, under the direction of David Wilber, M.D., medical director of Clinical Electrophysiology.
“Receiving IDE approval to begin clinical testing of our ablation therapy in patients is an important next step to advance treatment to reduce or potentially eliminate VT episodes,” said Michael Curley, Ph.D., FHRS, co-founder and CEO of Thermedical. “A successful therapy would significantly improve the quality-of-life for the more than 1 million people who develop, or are at high risk for developing VT annually.
Implantable cardioverter defibrillators are the current standard-of-care treatment for patients suffering from VT; however, this approach does not stop the progression of the disease or provide a cure. In addition, ICDs can be costly and painful, and may substantially reduce a patient’s quality of life. VT patients who have ICDs and recurrent VT may be treated today with conventional RF ablation, which is a lengthy procedure that has a success rate of approximately 50 percent.1
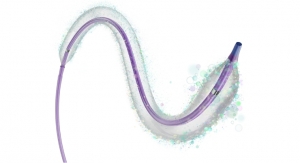
Thermedical’s patented ablation technology uses heat to kill the damaged heart muscle cells that are causing VT. The heat is delivered by the Durablate catheter, which simultaneously injects a precise flow of hot saline together with controlled RF energy into the heart tissue. This combination is an advanced form of biological heat transport that is 20 times more effective than conventional RF ablation methods. Compared to conventional VT ablation catheters, Durablate can more accurately control the ablation size, and it can treat tissue that is deeper in the heart wall, which is where life-threatening arrhythmias (abnormal, rapid heart rhythm) that cause VT are often located.
“Ablation therapy for the treatment of VT today is problematic, resulting in extremely long procedures and a low success rate,” said Dr. Curley. “There is significant need for an effective ablation therapy that can serve as an adjunct to, or as a possible replacement for ICDs. We believe our solution may be a low-cost alternative for treating VT, and it might save the U.S. healthcare system significant costs associated with VT treatment today.”
Thermedical is a privately held company founded by Massachusetts Institute of Technology (MIT) Hyperthermia Center alumni, Dr. Curley, and Patrick S. Hamilton, Ph.D., based in Waltham, Mass. Under a Massachusetts Life Sciences Center Small Business Matching Grant (SBMG) Award, multiple NIH* Small Business Innovation Research (SBIR) Grants, and Series A venture funding; the company has developed thermal-ablation systems to treat VT. The FDA-approved EFS will be the first human use of the Durablate Catheter Ablation System in the United States. Human studies began earlier in Canada and continue at three Canadian centers.
Reference
1. http://www.onlinejacc.org/content/accj/67/6/684.full.pdf
* Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R44HL132746. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The target population consists of patients who have already been treated with medicine, have an implantable cardioverter defibrillator (ICD), and who have had a conventional ablation procedure to treat VT, yet all their treatments have failed. The study is scheduled to begin in 2018 at Mayo Clinic under the direction of Douglas L. Packer, M.D., professor of medicine, and at Loyola University of Chicago Stritch School of Medicine, under the direction of David Wilber, M.D., medical director of Clinical Electrophysiology.
“Receiving IDE approval to begin clinical testing of our ablation therapy in patients is an important next step to advance treatment to reduce or potentially eliminate VT episodes,” said Michael Curley, Ph.D., FHRS, co-founder and CEO of Thermedical. “A successful therapy would significantly improve the quality-of-life for the more than 1 million people who develop, or are at high risk for developing VT annually.
Implantable cardioverter defibrillators are the current standard-of-care treatment for patients suffering from VT; however, this approach does not stop the progression of the disease or provide a cure. In addition, ICDs can be costly and painful, and may substantially reduce a patient’s quality of life. VT patients who have ICDs and recurrent VT may be treated today with conventional RF ablation, which is a lengthy procedure that has a success rate of approximately 50 percent.1
Thermedical’s patented ablation technology uses heat to kill the damaged heart muscle cells that are causing VT. The heat is delivered by the Durablate catheter, which simultaneously injects a precise flow of hot saline together with controlled RF energy into the heart tissue. This combination is an advanced form of biological heat transport that is 20 times more effective than conventional RF ablation methods. Compared to conventional VT ablation catheters, Durablate can more accurately control the ablation size, and it can treat tissue that is deeper in the heart wall, which is where life-threatening arrhythmias (abnormal, rapid heart rhythm) that cause VT are often located.
“Ablation therapy for the treatment of VT today is problematic, resulting in extremely long procedures and a low success rate,” said Dr. Curley. “There is significant need for an effective ablation therapy that can serve as an adjunct to, or as a possible replacement for ICDs. We believe our solution may be a low-cost alternative for treating VT, and it might save the U.S. healthcare system significant costs associated with VT treatment today.”
Thermedical is a privately held company founded by Massachusetts Institute of Technology (MIT) Hyperthermia Center alumni, Dr. Curley, and Patrick S. Hamilton, Ph.D., based in Waltham, Mass. Under a Massachusetts Life Sciences Center Small Business Matching Grant (SBMG) Award, multiple NIH* Small Business Innovation Research (SBIR) Grants, and Series A venture funding; the company has developed thermal-ablation systems to treat VT. The FDA-approved EFS will be the first human use of the Durablate Catheter Ablation System in the United States. Human studies began earlier in Canada and continue at three Canadian centers.
Reference
1. http://www.onlinejacc.org/content/accj/67/6/684.full.pdf
* Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R44HL132746. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.