Business Wire08.13.18
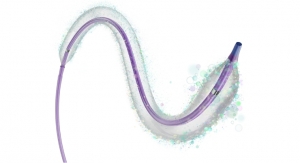
Axonics Modulation Technologies Inc., developer of the first rechargeable Sacral Neuromodulation (r-SNM) system for the treatment of urinary and bowel dysfunction, has received the CE mark for its Sacral Neuromodulation External Trial System.
Axonics received CE mark in June 2016 for its miniaturized implantable neurostimulator, tined lead, programmers, charger and related accessories. The External Trial System is an additional element of the Axonics r-SNM System used to help identify responders to Sacral Neuromodulation therapy prior to a permanent implant. It is composed of a temporary, single-use disposable external stimulator that is connected to either a Tined Lead or a temporary Peripheral Nerve Evaluation (PNE) Lead, depending on the preferred trial method.
This EU regulatory approval coincides with the commercial launch of the Axonics r-SNM System in the United Kingdom. Additional regulatory approvals in Europe are expected in the near term, including full-body magnetic resonance imaging conditional labeling.
Axonics is currently conducting a 120-patient pivotal clinical study under a U.S. Food and Drug Administration (FDA) Investigational Device Exemption for urinary dysfunction and has completed the enrollment and implant phase. The company anticipates FDA approval in the United States after the six-month post-implant endpoint has been reached for all patients and the FDA has completed its review of the pre-market approval application for the Axonics r-SNM System.
“To date, over 225 patients have been implanted with an Axonics r-SNM System in Europe and North America, the vast majority of which without undergoing an external trial period before permanent implant. We demonstrated that with quality lead placement and confirmation of intraoperative responses, outstanding clinical results can be achieved without an external trial,” said Raymond W. Cohen, CEO of Axonics. “However, from a commercial standpoint, having an External Trial System is necessary given that payors prefer to reimburse permanent implants after confirming that the patient is a trial responder. We are confident that Axonics is now well-positioned to capture significant worldwide share and, due to the benefits of our product offering, accelerate growth in the large $700 million Sacral Neuromodulation market.”
In April 2018, Axonics raised its latest round of capital to support commercialization activities in Europe and in the United States. Since March 2014, with the support of a number of venture firms located in the United States, France, the United Kingdom, the Netherlands, Switzerland and China, Axonics has raised over $114 million in equity capital in addition to a $20 million venture loan from Silicon Valley Bank.
Overactive bladder affects an estimated 85 million adults in the United States and Europe. Another 40 million are reported to suffer from fecal incontinence. SNM therapy is an effective and durable treatment that has been widely used and reimbursed in Europe and the United States for the past two decades. It is estimated that well over 300,000 patients have benefited from the therapy to date. SNM is the only OAB treatment with proven clinical superiority to standard medical therapy and OAB patients who receive SNM report significantly higher quality of life than patients undergoing drug treatment.
Axonics, based in Irvine, Calif., is a privately-held company that has developed an implantable Sacral Neuromodulation system for patients with urinary and bowel dysfunction. The company is focused on disrupting the SNM market that is currently dominated by one large provider. Annual sales are estimated at $700 million and is expected to grow to $1 billion by 2021. The Axonics r-SNM system includes a temporary disposable external trial system and long-lived implantable components such as a miniaturized rechargeable implantable stimulator qualified to function for at least 15 years, a tined lead, and patient-friendly items such as a charging system optimized for reduced charge time with no heating, a small patient remote control and an intuitive clinician programmer that facilitates lead placement procedure and programming.
Axonics received CE mark in June 2016 for its miniaturized implantable neurostimulator, tined lead, programmers, charger and related accessories. The External Trial System is an additional element of the Axonics r-SNM System used to help identify responders to Sacral Neuromodulation therapy prior to a permanent implant. It is composed of a temporary, single-use disposable external stimulator that is connected to either a Tined Lead or a temporary Peripheral Nerve Evaluation (PNE) Lead, depending on the preferred trial method.
This EU regulatory approval coincides with the commercial launch of the Axonics r-SNM System in the United Kingdom. Additional regulatory approvals in Europe are expected in the near term, including full-body magnetic resonance imaging conditional labeling.
Axonics is currently conducting a 120-patient pivotal clinical study under a U.S. Food and Drug Administration (FDA) Investigational Device Exemption for urinary dysfunction and has completed the enrollment and implant phase. The company anticipates FDA approval in the United States after the six-month post-implant endpoint has been reached for all patients and the FDA has completed its review of the pre-market approval application for the Axonics r-SNM System.
“To date, over 225 patients have been implanted with an Axonics r-SNM System in Europe and North America, the vast majority of which without undergoing an external trial period before permanent implant. We demonstrated that with quality lead placement and confirmation of intraoperative responses, outstanding clinical results can be achieved without an external trial,” said Raymond W. Cohen, CEO of Axonics. “However, from a commercial standpoint, having an External Trial System is necessary given that payors prefer to reimburse permanent implants after confirming that the patient is a trial responder. We are confident that Axonics is now well-positioned to capture significant worldwide share and, due to the benefits of our product offering, accelerate growth in the large $700 million Sacral Neuromodulation market.”
In April 2018, Axonics raised its latest round of capital to support commercialization activities in Europe and in the United States. Since March 2014, with the support of a number of venture firms located in the United States, France, the United Kingdom, the Netherlands, Switzerland and China, Axonics has raised over $114 million in equity capital in addition to a $20 million venture loan from Silicon Valley Bank.
Overactive bladder affects an estimated 85 million adults in the United States and Europe. Another 40 million are reported to suffer from fecal incontinence. SNM therapy is an effective and durable treatment that has been widely used and reimbursed in Europe and the United States for the past two decades. It is estimated that well over 300,000 patients have benefited from the therapy to date. SNM is the only OAB treatment with proven clinical superiority to standard medical therapy and OAB patients who receive SNM report significantly higher quality of life than patients undergoing drug treatment.
Axonics, based in Irvine, Calif., is a privately-held company that has developed an implantable Sacral Neuromodulation system for patients with urinary and bowel dysfunction. The company is focused on disrupting the SNM market that is currently dominated by one large provider. Annual sales are estimated at $700 million and is expected to grow to $1 billion by 2021. The Axonics r-SNM system includes a temporary disposable external trial system and long-lived implantable components such as a miniaturized rechargeable implantable stimulator qualified to function for at least 15 years, a tined lead, and patient-friendly items such as a charging system optimized for reduced charge time with no heating, a small patient remote control and an intuitive clinician programmer that facilitates lead placement procedure and programming.