LivaNova plc07.20.18
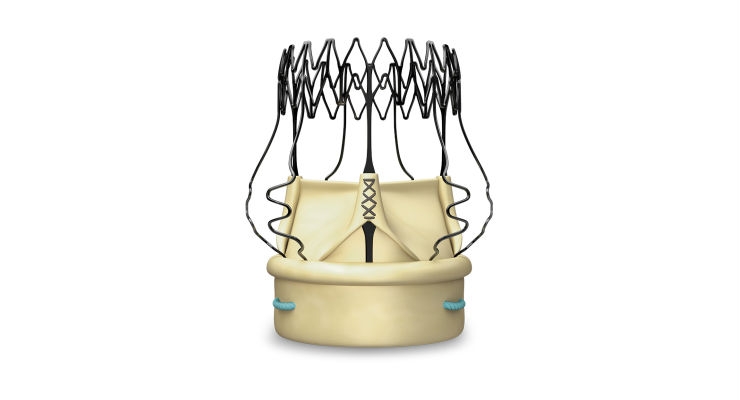
LivaNova PLC announced that Japan’s Ministry of Health, Labour and Welfare has approved the company’s Perceval sutureless aortic heart valve to treat aortic valve disease.
“With this approval for Perceval, an innovative and trusted valve platform, we are able to provide patients and clinicians in Japan with a new option for aortic heart valve replacement,” said Noriaki Kawana, president of LivaNova Japan.
The Perceval sutureless valve is supported by a strong body of evidence with more than 10 years of clinical experience. The valve is specifically designed to reduce the physiological impact of aortic valve replacement and improve patient outcomes. Perceval is also designed to be highly versatile and suitable for a wide range of surgical approaches, including traditional and minimally invasive.
“At LivaNova, we are committed to enhancing patient care around the world, and will continue to address the increasing global need for advanced aortic valve solutions,” said Roy Khoury, president of LivaNova International.
LivaNova obtained CE Mark for Perceval in 2011 and U.S. Food and Drug Administration approval in 2016. To date, more than 25,000 patients worldwide have been treated with the Perceval valve.
LivaNova PLC is a global medical technology company built on nearly five decades of experience and a commitment to improve the lives of patients worldwide. LivaNova’s technologies and treatments provide meaningful solutions for the benefit of patients, healthcare professionals and healthcare systems. Headquartered in London, LivaNova has a presence in more than 100 countries worldwide. The company currently employs more than 3,500 employees. LivaNova operates as two businesses: Cardiac Surgery and Neuromodulation, with operating headquarters in Mirandola, Italy; and Houston, respectively.
“With this approval for Perceval, an innovative and trusted valve platform, we are able to provide patients and clinicians in Japan with a new option for aortic heart valve replacement,” said Noriaki Kawana, president of LivaNova Japan.
The Perceval sutureless valve is supported by a strong body of evidence with more than 10 years of clinical experience. The valve is specifically designed to reduce the physiological impact of aortic valve replacement and improve patient outcomes. Perceval is also designed to be highly versatile and suitable for a wide range of surgical approaches, including traditional and minimally invasive.
“At LivaNova, we are committed to enhancing patient care around the world, and will continue to address the increasing global need for advanced aortic valve solutions,” said Roy Khoury, president of LivaNova International.
LivaNova obtained CE Mark for Perceval in 2011 and U.S. Food and Drug Administration approval in 2016. To date, more than 25,000 patients worldwide have been treated with the Perceval valve.
LivaNova PLC is a global medical technology company built on nearly five decades of experience and a commitment to improve the lives of patients worldwide. LivaNova’s technologies and treatments provide meaningful solutions for the benefit of patients, healthcare professionals and healthcare systems. Headquartered in London, LivaNova has a presence in more than 100 countries worldwide. The company currently employs more than 3,500 employees. LivaNova operates as two businesses: Cardiac Surgery and Neuromodulation, with operating headquarters in Mirandola, Italy; and Houston, respectively.