Business Wire07.02.18
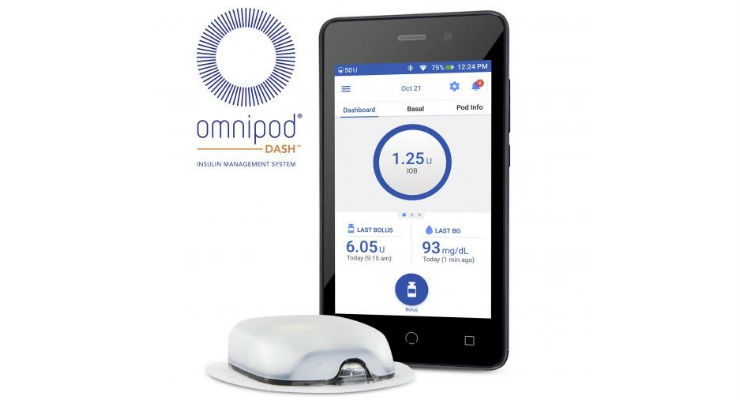
Insulet Corporation, a provider of tubeless insulin pump technology via its Omnipod Insulin Management System, has announced as of July 1, it has assumed direct commercial operations in Europe to provide sales, distribution, customer support, and product services for its Omnipod System. Insulet has established a dedicated Customer Care team, committed to providing best-in-class support to its large European customer base.
“We are thrilled to directly support the diabetes community across Europe now and into the future,” said Patrick Sullivan, chairman and chief executive officer. “We have a very accomplished team in place whose sole focus is to deliver an exceptional customer experience and we are deeply committed to bringing customized innovation to the local European markets.”
Since July 2017, when Insulet announced its intention to assume the distribution, sales, marketing, training, and support activities for the Omnipod System across Europe, the company has established a highly-talented European team of over 100 employees with extensive diabetes knowledge and expertise within the European markets. In addition, Insulet now has 24/7 customer care and product support for over 140,000 Podders worldwide.
“Insulet has always been committed to putting the patient first and direct operations in Europe is a big step forward for all Omnipod users,” said Professor Eric Renard, professor of endocrinology, Lapeyronie University Hospital, Montpellier, France. “Insulet is making a significant, long-term commitment to the diabetes community in Europe and I’m excited they plan to bring new innovations to market that best serve the local European customers.”
For more information on the Omnipod System, visit the company’s Omnipod website.
“We are thrilled to directly support the diabetes community across Europe now and into the future,” said Patrick Sullivan, chairman and chief executive officer. “We have a very accomplished team in place whose sole focus is to deliver an exceptional customer experience and we are deeply committed to bringing customized innovation to the local European markets.”
Since July 2017, when Insulet announced its intention to assume the distribution, sales, marketing, training, and support activities for the Omnipod System across Europe, the company has established a highly-talented European team of over 100 employees with extensive diabetes knowledge and expertise within the European markets. In addition, Insulet now has 24/7 customer care and product support for over 140,000 Podders worldwide.
“Insulet has always been committed to putting the patient first and direct operations in Europe is a big step forward for all Omnipod users,” said Professor Eric Renard, professor of endocrinology, Lapeyronie University Hospital, Montpellier, France. “Insulet is making a significant, long-term commitment to the diabetes community in Europe and I’m excited they plan to bring new innovations to market that best serve the local European customers.”
For more information on the Omnipod System, visit the company’s Omnipod website.