Business Wire06.20.18
EchoNous, a developer of intelligent medical tools, announces that Michael Blaivas, M.D., MBA, is now serving as its chief medical officer (CMO). Blaivas is a board-certified emergency medicine physician and a recognized pioneer in emergency medicine and point-of-care ultrasound education. Blaivas’ offers broad expertise in the application of point-of-care ultrasound practices and will help ensure EchoNous’ family of intelligent medical tools are developed toward industry-changing innovations that improve patient care and drive significant efficiencies in the healthcare system.
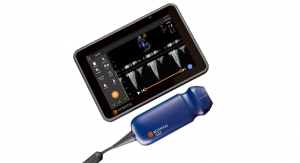
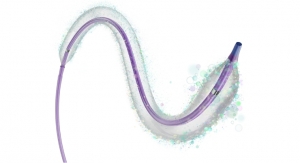
As CMO, Blaivas’ main focuses will be to help lead the company’s ongoing development of a transcendent new intelligent medical tool for heart and lung assessment, where he is a recognized authority in lung ultrasound, while contributing to ongoing improvement of the company’s soon-to-be released EchoNous Vein1—a new tool designed to improve first-time peripheral IV placement.
“Dr. Blaivas is a true ‘original’ in the use of emergency medicine ultrasound and point-of-care ultrasound education,” said Kevin Goodwin, CEO of EchoNous. “Mike is someone I have observed for nearly 19 years, having him onboard as our chief medical officer will further tighten our ongoing execution on our vision to solve common everyday problems in healthcare by converging emerging AI methods with our world-leading ultrasound miniaturization skills.”
Blaivas is affiliate professor of Medicine, University of South Carolina School of Medicine, who has published more than 160 peer-reviewed articles on point-of-care ultrasound and edited five books. He is a founding member of WINFOCUS (The World Interactive Network Focused On Critical UltraSound) and was its second president. Blaivas was also a founding member and second president of SUSME (Society of Ultrasound in Medical Education). Previously, he was chairman of the emergency ultrasound section for American College of Emergency Physicians.
“This opportunity truly excites me with my passion for the power of ultrasound in point-of-care medicine, and EchoNous’ approach to using AI to directly improve patient care and clinician performance,” said Blaivas. “I have known and respected Kevin Goodwin as a pioneer in the field of point-of-care ultrasound and now more recently intelligent ultrasound-based tools. I’m hugely excited to join EchoNous at a time when the company is blazing a trail in a healthcare system in need of efficient and revolutionary solutions to the age-old challenges of care and cost.”
Blaivas has a Doctor of Medicine from the University of Michigan, an MBA in management of technology from Georgia Tech University, and a certificate in artificial intelligence from the Massachusetts Institute of Technology Sloan School of Management.
Headquartered in Seattle, Wash., EchoNous, a KKR portfolio company and parent company of Signostics, is developing an expanding family of intelligent medical tools. Beginning with the soon-to-be-released EchoNous Vein vascular access tool (pending U.S. Food and Drug Administration approval) and the Uscan intelligent bladder scanner, EchoNous is applying a layer of artificial intelligence methods with the extreme miniaturized ultrasound technology to provide nurses, doctors and clinicians with tools simplifying the task at hand.
Reference
1. This device is currently 510(k) pending. The information contained herein is provided for informational purposes only. The device is not for sale and we are not currently accepting any orders.
As CMO, Blaivas’ main focuses will be to help lead the company’s ongoing development of a transcendent new intelligent medical tool for heart and lung assessment, where he is a recognized authority in lung ultrasound, while contributing to ongoing improvement of the company’s soon-to-be released EchoNous Vein1—a new tool designed to improve first-time peripheral IV placement.
“Dr. Blaivas is a true ‘original’ in the use of emergency medicine ultrasound and point-of-care ultrasound education,” said Kevin Goodwin, CEO of EchoNous. “Mike is someone I have observed for nearly 19 years, having him onboard as our chief medical officer will further tighten our ongoing execution on our vision to solve common everyday problems in healthcare by converging emerging AI methods with our world-leading ultrasound miniaturization skills.”
Blaivas is affiliate professor of Medicine, University of South Carolina School of Medicine, who has published more than 160 peer-reviewed articles on point-of-care ultrasound and edited five books. He is a founding member of WINFOCUS (The World Interactive Network Focused On Critical UltraSound) and was its second president. Blaivas was also a founding member and second president of SUSME (Society of Ultrasound in Medical Education). Previously, he was chairman of the emergency ultrasound section for American College of Emergency Physicians.
“This opportunity truly excites me with my passion for the power of ultrasound in point-of-care medicine, and EchoNous’ approach to using AI to directly improve patient care and clinician performance,” said Blaivas. “I have known and respected Kevin Goodwin as a pioneer in the field of point-of-care ultrasound and now more recently intelligent ultrasound-based tools. I’m hugely excited to join EchoNous at a time when the company is blazing a trail in a healthcare system in need of efficient and revolutionary solutions to the age-old challenges of care and cost.”
Blaivas has a Doctor of Medicine from the University of Michigan, an MBA in management of technology from Georgia Tech University, and a certificate in artificial intelligence from the Massachusetts Institute of Technology Sloan School of Management.
Headquartered in Seattle, Wash., EchoNous, a KKR portfolio company and parent company of Signostics, is developing an expanding family of intelligent medical tools. Beginning with the soon-to-be-released EchoNous Vein vascular access tool (pending U.S. Food and Drug Administration approval) and the Uscan intelligent bladder scanner, EchoNous is applying a layer of artificial intelligence methods with the extreme miniaturized ultrasound technology to provide nurses, doctors and clinicians with tools simplifying the task at hand.
Reference
1. This device is currently 510(k) pending. The information contained herein is provided for informational purposes only. The device is not for sale and we are not currently accepting any orders.