Business Wire04.30.18
Tandem Diabetes Care Inc., a medical device company and manufacturer of the only touchscreen insulin pumps, today announced receipt of CE mark approval for the t:slim X2 Insulin Pump with Dexcom G5 Mobile continuous glucose monitoring (CGM) integration1. The Company plans to begin commercial sales of the pump in select international markets beginning in the second half of 2018. The company has been commercializing its touchscreen insulin pumps in the United States since 2012, and received U.S. Food and Drug Administration (FDA) approval of the t:slim X2 Insulin Pump with Dexcom G5 Mobile CGM integration in August 2017.
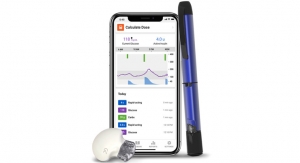
The simple-to-use t:slim X2 Insulin Pump includes advanced features like a large color touchscreen, rechargeable battery, USB connectivity and watertight construction (IPX7)2. It is the only pump that integrates with Dexcom G5 Mobile CGM, and the first CGM-enabled pump approved to let users make treatment decisions without pricking their finger.3 The t:slim X2 Pump is up to 38 percent smaller than other insulin pumps and holds up to 300 units of insulin.4
“As our first product to receive CE mark, this milestone is key to the fulfillment of our vision to bring the features and benefits of our unique insulin pump technology to people outside of the United States,” said Kim Blickenstaff, President and CEO of Tandem Diabetes Care. “Our plans to launch the t:slim X2 Pump internationally in the second half of this year is of particular strategic importance during this time where insulin pump options are becoming increasingly limited."
References
1Dexcom G5 Mobile CGM sold separately.
2Tested to a depth of 3 feet for 30 minutes
3CGM-based treatment requires fingersticks for calibration; may result in hypoglycemia if calibration not performed, when taking acetaminophen, or if symptoms/expectations do not match CGM readings.
438 percent smaller than MiniMed 630G and 670G and at least 28 percent smaller than MiniMed 530G, Animas Vibe and Omnipod System. Data on file, Tandem Diabetes Care.
The simple-to-use t:slim X2 Insulin Pump includes advanced features like a large color touchscreen, rechargeable battery, USB connectivity and watertight construction (IPX7)2. It is the only pump that integrates with Dexcom G5 Mobile CGM, and the first CGM-enabled pump approved to let users make treatment decisions without pricking their finger.3 The t:slim X2 Pump is up to 38 percent smaller than other insulin pumps and holds up to 300 units of insulin.4
“As our first product to receive CE mark, this milestone is key to the fulfillment of our vision to bring the features and benefits of our unique insulin pump technology to people outside of the United States,” said Kim Blickenstaff, President and CEO of Tandem Diabetes Care. “Our plans to launch the t:slim X2 Pump internationally in the second half of this year is of particular strategic importance during this time where insulin pump options are becoming increasingly limited."
References
1Dexcom G5 Mobile CGM sold separately.
2Tested to a depth of 3 feet for 30 minutes
3CGM-based treatment requires fingersticks for calibration; may result in hypoglycemia if calibration not performed, when taking acetaminophen, or if symptoms/expectations do not match CGM readings.
438 percent smaller than MiniMed 630G and 670G and at least 28 percent smaller than MiniMed 530G, Animas Vibe and Omnipod System. Data on file, Tandem Diabetes Care.