Business Wire03.27.18
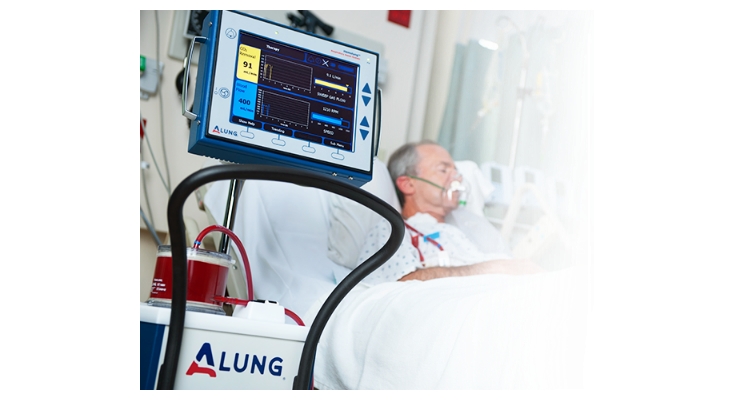
ALung Technologies Inc. has initiated patient enrollment in its VENT-AVOID Trial of the Hemolung RAS. The Minneapolis Heart Institute Foundation recently enrolled the first study subject at Abbott Northwestern Hospital in Minneapolis, Minn. The VENT-AVOID Trial is the world’s first pivotal trial of extracorporeal carbon dioxide removal (ECCO2R) for treating patients with COPD exacerbations. The study is being conducted under an Investigational Device Exemption (IDE) which ALung received from the U.S. Food and Drug Administration (FDA) in late 2017.
“We are excited to be working with institutions like Minneapolis Heart Institute Foundation to enroll patients in the VENT-AVOID Trial,” said Peter DeComo, chairman and CEO of ALung. “We are very grateful for their efforts to enroll their first patient and look forward to their continued participation in the study.”
The VENT-AVOID Trial is a prospective, multi-center, randomized, controlled, pivotal trial to validate the safety and efficacy of the Hemolung Respiratory Assist System for COPD patients experiencing an acute exacerbation requiring ventilatory support. COPD patients suffering severe exacerbations are potentially eligible for the study if they are either 1) failing non-invasive ventilation and presenting a high risk of being intubated and mechanically ventilated or 2) have required intubation and invasive mechanical ventilation due to acute respiratory failure.
Leading the study at Minneapolis Heart Institute Foundation is Dr. Ramiro Saavedra-Romero, an intensivist and director of Medical ECMO Program, and medical director of MICU/SICU at Abbott Northwestern Hospital. “We’re very proud to be the first study site to enroll in the VENT-AVOID Trial,” noted Dr. Saavedra-Romero. “I see the promise in extracorporeal carbon dioxide removal as a new and safe treatment modality when traditional methods of ventilatory support are failing or deemed undesirable.”
COPD affects 30 million Americans1 and is the third leading cause of death in the United States behind cancer and heart disease.2 Acute exacerbations, defined as a sudden worsening of COPD symptoms, are a major cause of morbidity and mortality in COPD patients. For patients with severe exacerbations, high levels of carbon dioxide can result in respiratory failure and the need for intubation and mechanical ventilation as life saving measures. Unfortunately, mechanical ventilation is associated with many side effects, and in-hospital mortality remains as high as 30 percent.3 ECCO2R therapy with the Hemolung RAS allows carbon dioxide to be removed from the blood independently of the lungs with the aim of facilitating the avoidance or reduction of intubation and invasive mechanical ventilation.
In addition to the US-based VENT-AVOID study, The Hemolung RAS is also being studied in a landmark pivotal study for patients with acute respiratory distress syndrome (ARDS) as part of the 1,120-patient REST Trial in the United Kingdom. Nearly 200 patients have been enrolled in the REST Trial, making it the largest trial of ECCO2R to date. ALung is the only company pursuing two major pivotal trials to validate the safety and efficacy of extracorporeal carbon dioxide removal therapy.
ALung Technologies Inc. is a privately-held Pittsburgh, Pa.-based developer and manufacturer of innovative lung assist devices. Founded in 1997 as a spin-out of the University of Pittsburgh, ALung has developed the Hemolung RAS as a dialysis-like alternative or supplement to mechanical ventilation. ALung is backed by Philips, UPMC Enterprises, Abiomed, The Accelerator Fund, Allos Ventures, Birchmere Ventures, Blue Tree Ventures, Eagle Ventures, Riverfront Ventures, West Capital Advisors, and other individual investors.
The Hemolung RAS is an investigational device and limited by United States law to investigational use.
The Minneapolis Heart Institute Foundation (MHIF) is a recognized research leader in cardiovascular medicine and population health initiatives. Each year MHIF leads more than 175 active research projects and publishes more than 175 peer-reviewed abstracts. MHIF provides more than 10,000 hours of education each year putting its research into practice to improve outcomes.
References
1. https://www.copdfoundation.org/What-is-COPD/COPD-Facts/Statistics.aspx
2. http://www.lung.org/assets/documents/research/copd-trend-report.pdf
3. Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. Jan 15 2012;185(2):152-159.
“We are excited to be working with institutions like Minneapolis Heart Institute Foundation to enroll patients in the VENT-AVOID Trial,” said Peter DeComo, chairman and CEO of ALung. “We are very grateful for their efforts to enroll their first patient and look forward to their continued participation in the study.”
The VENT-AVOID Trial is a prospective, multi-center, randomized, controlled, pivotal trial to validate the safety and efficacy of the Hemolung Respiratory Assist System for COPD patients experiencing an acute exacerbation requiring ventilatory support. COPD patients suffering severe exacerbations are potentially eligible for the study if they are either 1) failing non-invasive ventilation and presenting a high risk of being intubated and mechanically ventilated or 2) have required intubation and invasive mechanical ventilation due to acute respiratory failure.
Leading the study at Minneapolis Heart Institute Foundation is Dr. Ramiro Saavedra-Romero, an intensivist and director of Medical ECMO Program, and medical director of MICU/SICU at Abbott Northwestern Hospital. “We’re very proud to be the first study site to enroll in the VENT-AVOID Trial,” noted Dr. Saavedra-Romero. “I see the promise in extracorporeal carbon dioxide removal as a new and safe treatment modality when traditional methods of ventilatory support are failing or deemed undesirable.”
COPD affects 30 million Americans1 and is the third leading cause of death in the United States behind cancer and heart disease.2 Acute exacerbations, defined as a sudden worsening of COPD symptoms, are a major cause of morbidity and mortality in COPD patients. For patients with severe exacerbations, high levels of carbon dioxide can result in respiratory failure and the need for intubation and mechanical ventilation as life saving measures. Unfortunately, mechanical ventilation is associated with many side effects, and in-hospital mortality remains as high as 30 percent.3 ECCO2R therapy with the Hemolung RAS allows carbon dioxide to be removed from the blood independently of the lungs with the aim of facilitating the avoidance or reduction of intubation and invasive mechanical ventilation.
In addition to the US-based VENT-AVOID study, The Hemolung RAS is also being studied in a landmark pivotal study for patients with acute respiratory distress syndrome (ARDS) as part of the 1,120-patient REST Trial in the United Kingdom. Nearly 200 patients have been enrolled in the REST Trial, making it the largest trial of ECCO2R to date. ALung is the only company pursuing two major pivotal trials to validate the safety and efficacy of extracorporeal carbon dioxide removal therapy.
ALung Technologies Inc. is a privately-held Pittsburgh, Pa.-based developer and manufacturer of innovative lung assist devices. Founded in 1997 as a spin-out of the University of Pittsburgh, ALung has developed the Hemolung RAS as a dialysis-like alternative or supplement to mechanical ventilation. ALung is backed by Philips, UPMC Enterprises, Abiomed, The Accelerator Fund, Allos Ventures, Birchmere Ventures, Blue Tree Ventures, Eagle Ventures, Riverfront Ventures, West Capital Advisors, and other individual investors.
The Hemolung RAS is an investigational device and limited by United States law to investigational use.
The Minneapolis Heart Institute Foundation (MHIF) is a recognized research leader in cardiovascular medicine and population health initiatives. Each year MHIF leads more than 175 active research projects and publishes more than 175 peer-reviewed abstracts. MHIF provides more than 10,000 hours of education each year putting its research into practice to improve outcomes.
References
1. https://www.copdfoundation.org/What-is-COPD/COPD-Facts/Statistics.aspx
2. http://www.lung.org/assets/documents/research/copd-trend-report.pdf
3. Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. Jan 15 2012;185(2):152-159.