Business Wire03.20.18
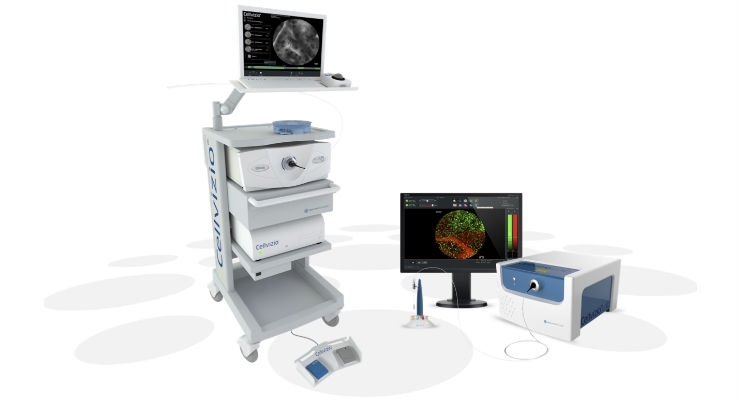
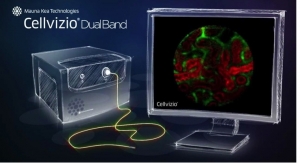
Cook Medical’s Cellvizio Confocal Laser Endomicroscopy (CLE) System and the Confocal Miniprobes have received additional 510(k) clearance from the U.S. Food and Drug Administration (FDA). Additional data provided in multiple peer-reviewed medical journals paved the way for this new clearance and indication for the Cellvizio system. This new indication states that it can be used for in vivo imaging of the internal microstructure of tissues, including the identification of cells and vessels and their organization or architecture during endoscopic procedures.
“We are excited for this new clearance for Cellvizio and what it means for the patients we serve,” said DJ Sirota, vice president of Cook Medical. “The ability to identify cells and vessels and their organization or architecture should have a significant impact on the use of this system with urologists, and we are excited to work with Mauna Kea to bring this technology to the urology specialty.”
Cellvizio is a probe-based CLE system, a visualization platform that incorporates laser scanning technology to generate images of the internal microstructure of tissues. The Confocal Miniprobes are made up of optical fiber bundles and other optical elements. They transmit the laser light to the tissue site and detect the fluorescence signal that is emitted back, which is turned into real-time images of the internal microstructure of tissues.
Manufactured by Mauna Kea Technologies, the Cellvizio system and Confocal Miniprobes are available through distribution by Cook Medical.
“We are excited for this new clearance for Cellvizio and what it means for the patients we serve,” said DJ Sirota, vice president of Cook Medical. “The ability to identify cells and vessels and their organization or architecture should have a significant impact on the use of this system with urologists, and we are excited to work with Mauna Kea to bring this technology to the urology specialty.”
Cellvizio is a probe-based CLE system, a visualization platform that incorporates laser scanning technology to generate images of the internal microstructure of tissues. The Confocal Miniprobes are made up of optical fiber bundles and other optical elements. They transmit the laser light to the tissue site and detect the fluorescence signal that is emitted back, which is turned into real-time images of the internal microstructure of tissues.
Manufactured by Mauna Kea Technologies, the Cellvizio system and Confocal Miniprobes are available through distribution by Cook Medical.