Business Wire03.05.18
Somahlution, a global biotechnology company developing products to reduce the burden of ischemia reperfusion injury in tissue grafting, organ transplant and other surgical indications, announced that Golden Jubilee National Hospital and Victoria Blackpool Hospital in the United Kingdom have initiated patient enrollment in the DuraGraft European Registry clinical trial. The registry will evaluate the benefits of treatment with DuraGraft, a one-time intraoperative treatment designed to prevent vascular graft disease and graft failure and reduce the clinical complications associated with graft failure in patients treated with coronary artery bypass grafting (CABG) surgery.
“We are very excited to be one of the first clinical sites in the United Kingdom to initiate enrollment of patients in the DuraGraft European Registry,” said Amal K. Bose, M.D., cardiothoracic surgeon at Lancashire Cardiac Centre at Victoria Blackpool Hospital. “DuraGraft has the potential to provide significant improvements in graft patency with benefits for both surgeons and more importantly CABG patients.”
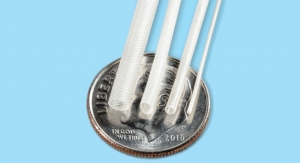
When patients with heart disease undergo CABG surgery, a vein graft is often removed from the leg, wrist, or chest and transplanted to the heart. In many cases, in the minutes between removal and implantation, the graft is stored in the operating room without circulating blood or oxygen supply, potentially increasing the risk of ischemia reperfusion injury and graft damage. DuraGraft is a pH and ionically balanced solution containing salts, antioxidants and other important components to protect against ischemia reperfusion injury in which the graft may be safely preserved and stored prior to implantation.
“In many cases, vascular conduits can be at risk during CABG surgery,” said Nawwar Al-Attar, FRCS, FETCS, Ph.D., consultant cardiac and transplant surgeon and director of transplant service at Scotland’s Golden Jubilee National Hospital. “A growing body of evidence shows that the DuraGraft solution may help preserve and store vascular grafts in the operating room prior to implantation and potentially prevent graft rejection. We are looking forward to participating in this important research with the University of Glasgow and to the prospect of bringing this innovative technology to appropriate CABG patients in the years ahead.”
Patients undergoing CABG-only or CABG-plus valve surgery will be consented to participate in the DuraGraft European Registry. For these patients, baseline clinical and angiographic characteristics as well as certain procedural and post-op clinical events will be recorded. Clinical outcomes will be assessed post-CABG surgery through hospitalization at 30 days and annually up to five years.
“As more leading hospitals and surgeons across Europe join this important registry, we believe the collected data will provide further supporting evidence reinforcing the importance of appropriate graft treatment during surgery and the role of DuraGraft in improving outcomes,” said Maximilian Y. Emmert, M.D., Ph.D., professor at the University of Zurich and the registry lead principal investigator.
“We are pleased to initiate this European Registry in the UK,” said Tracy Goeken, M.D., chief medical offer at Somahlution. “The DuraGraft Registry meets one of our post marketing commitments to our European Regulatory Authority and will collect important, real-world clinical data on the application of this technology, as a one-time intraoperative treatment in CABG surgery. Our goal is to improve the standard of care for thousands of patients who receive CABG surgery in the United Kingdom and around the world each year.”
DuraGraft is the first commercial product based on proprietary technology platform. DuraGraft is CE Marked in Europe and available in other global markets for CABG and peripheral bypass indications, and is not yet commercially available in the United States.
Somahlution develops products to reduce the burden of ischemia reperfusion injury in tissue grafting, organ transplant and other surgical indications. The company’s flagship product, DuraGraft, is a vascular graft treatment that improves clinical outcomes by reducing the incidence of complications associated with graft failure. DuraGraft enhances CABG surgical outcomes by significantly reducing major cardiac events such as repeat revascularization and myocardial infarction.
Blackpool Victoria Hospital is a site of Blackpool Teaching Hospitals NHS Foundation Trust. The Trust is situated on the west coast of Lancashire, United Kingdom, and operates within a regional health economy catchment area that spans Lancashire and South Cumbria, supporting a population of 1.6 million. The Trust is a provider of specialist tertiary care for cardiac and haematology services across this region. The Trust does not operate outside of the United Kingdom.
The Trust provides a range of acute services to the 330,000 population of the Fylde Coast health economy and the estimated 11 million visitors to the seaside town of Blackpool. Since April 1, 2012, the Trust also provides a wide range of community health services to the 440,000 residents of Blackpool, Fylde, Wyre and North Lancashire. The Trust also hosts the National Artificial Eye Service, which provides services across England.
A national institution, independently run by its own NHS Board, the Golden Jubilee Foundation is helping to redefine the concept of the public hospital, with a vision of ‘Leading quality, research and innovation’ for NHSScotland. The Golden Jubilee Foundation family includes Golden Jubilee National Hospital; Golden Jubilee Research Institute; Golden Jubilee Innovation Centre; and Golden Jubilee Conference Hotel.
“We are very excited to be one of the first clinical sites in the United Kingdom to initiate enrollment of patients in the DuraGraft European Registry,” said Amal K. Bose, M.D., cardiothoracic surgeon at Lancashire Cardiac Centre at Victoria Blackpool Hospital. “DuraGraft has the potential to provide significant improvements in graft patency with benefits for both surgeons and more importantly CABG patients.”
When patients with heart disease undergo CABG surgery, a vein graft is often removed from the leg, wrist, or chest and transplanted to the heart. In many cases, in the minutes between removal and implantation, the graft is stored in the operating room without circulating blood or oxygen supply, potentially increasing the risk of ischemia reperfusion injury and graft damage. DuraGraft is a pH and ionically balanced solution containing salts, antioxidants and other important components to protect against ischemia reperfusion injury in which the graft may be safely preserved and stored prior to implantation.
“In many cases, vascular conduits can be at risk during CABG surgery,” said Nawwar Al-Attar, FRCS, FETCS, Ph.D., consultant cardiac and transplant surgeon and director of transplant service at Scotland’s Golden Jubilee National Hospital. “A growing body of evidence shows that the DuraGraft solution may help preserve and store vascular grafts in the operating room prior to implantation and potentially prevent graft rejection. We are looking forward to participating in this important research with the University of Glasgow and to the prospect of bringing this innovative technology to appropriate CABG patients in the years ahead.”
Patients undergoing CABG-only or CABG-plus valve surgery will be consented to participate in the DuraGraft European Registry. For these patients, baseline clinical and angiographic characteristics as well as certain procedural and post-op clinical events will be recorded. Clinical outcomes will be assessed post-CABG surgery through hospitalization at 30 days and annually up to five years.
“As more leading hospitals and surgeons across Europe join this important registry, we believe the collected data will provide further supporting evidence reinforcing the importance of appropriate graft treatment during surgery and the role of DuraGraft in improving outcomes,” said Maximilian Y. Emmert, M.D., Ph.D., professor at the University of Zurich and the registry lead principal investigator.
“We are pleased to initiate this European Registry in the UK,” said Tracy Goeken, M.D., chief medical offer at Somahlution. “The DuraGraft Registry meets one of our post marketing commitments to our European Regulatory Authority and will collect important, real-world clinical data on the application of this technology, as a one-time intraoperative treatment in CABG surgery. Our goal is to improve the standard of care for thousands of patients who receive CABG surgery in the United Kingdom and around the world each year.”
DuraGraft is the first commercial product based on proprietary technology platform. DuraGraft is CE Marked in Europe and available in other global markets for CABG and peripheral bypass indications, and is not yet commercially available in the United States.
Somahlution develops products to reduce the burden of ischemia reperfusion injury in tissue grafting, organ transplant and other surgical indications. The company’s flagship product, DuraGraft, is a vascular graft treatment that improves clinical outcomes by reducing the incidence of complications associated with graft failure. DuraGraft enhances CABG surgical outcomes by significantly reducing major cardiac events such as repeat revascularization and myocardial infarction.
Blackpool Victoria Hospital is a site of Blackpool Teaching Hospitals NHS Foundation Trust. The Trust is situated on the west coast of Lancashire, United Kingdom, and operates within a regional health economy catchment area that spans Lancashire and South Cumbria, supporting a population of 1.6 million. The Trust is a provider of specialist tertiary care for cardiac and haematology services across this region. The Trust does not operate outside of the United Kingdom.
The Trust provides a range of acute services to the 330,000 population of the Fylde Coast health economy and the estimated 11 million visitors to the seaside town of Blackpool. Since April 1, 2012, the Trust also provides a wide range of community health services to the 440,000 residents of Blackpool, Fylde, Wyre and North Lancashire. The Trust also hosts the National Artificial Eye Service, which provides services across England.
A national institution, independently run by its own NHS Board, the Golden Jubilee Foundation is helping to redefine the concept of the public hospital, with a vision of ‘Leading quality, research and innovation’ for NHSScotland. The Golden Jubilee Foundation family includes Golden Jubilee National Hospital; Golden Jubilee Research Institute; Golden Jubilee Innovation Centre; and Golden Jubilee Conference Hotel.