Business Wire01.08.18
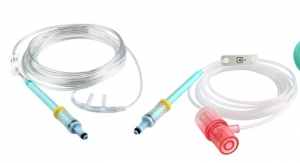
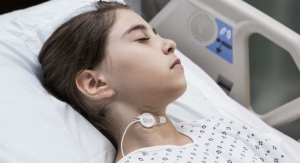
Rhythmlink International LLC has released a new product line designed to maximize efficiency and workflow when recording EEG on patients who require magnetic resonance (MR) or computed tomography (CT) imaging. The Quick Connect System is a line of disposable EEG electrodes arranged in multiple electrode arrays, resulting in fewer connection points and easier organization of lead wires at bedside. The launch includes a total of 18 new product numbers, all of which are a part of Rhythmlink’s MR Conditional/CT electrodes, which are the only U.S. Food and Drug Administration (FDA)-cleared electrodes on the market for 1.5 and 3T scanners.
The Quick Connect System offers the same superior recording quality of Rhythmlink’s original MR Conditional/CT electrodes, allowing electrodes to remain on the patient while in a 1.5T and 3T MR scanner. Once imaging is complete, patients are quickly and efficiently reconnected to their monitoring device via Rhythmlink’s new Quick Connect System, resulting in both time and costs saved and improved patient care.
“We are extremely proud to be releasing the new Quick Connect System,” said Shawn Regan, CEO of Rhythmlink International. “Since releasing the first FDA Cleared MR Conditional electrodes several years ago, our team has been working hard to improve the product, and to reach the next step in efficient patient care. We believe the Quick Connect System will meet a need in healthcare that many have been waiting for.”
Each new product number features numbered heat shrink and flags designed to assist bedside personnel such as nursing teams to easily disconnect and reconnect patients without removing the EEG electrodes when the patient requires a CT or magnetic resonance imaging (MRI). Electrodes are available in Slim Cup, a Deep Cup design that holds more gel, and a Webb design that provides a larger recording surface. All three designs can be mixed to help reduce skin breakdown and increase patient comfort, and custom array options will be available in the future to meet additional patient needs.
“These products offer the next level of high efficiency workflow,” Vice President of Global Sales Leah Hanson, R.EEG/EP T., said. “The Quick Connect System not only eliminates the need for removal and reapplication of EEG electrodes when a patient needs a CT or MRI, but reduces the overall time of the disconnection and reconnection process. It demonstrates that at Rhythmlink we understand that every moment counts when it comes to patient care.”
The new Quick Connect System adds to their extensive product lines focused on brain health, routine EEG monitoring, ICU, and more.
Rhythmlink International is a medical device manufacturing company specializing in devices that help connect patients to machines to record or elicit physiologic information. Rhythmlink designs, manufactures and distributes a variety of medical devices for intraoperative neuromonitoring, electroencephalography, evoked potentials, polysomnography, long-term monitoring epilepsy and critical care units. Founded by neurodiagnostic technicians and engineers in 2002, Rhythmlink also offers custom packaging, custom products, private labeling and contract manufacturing services.
The Quick Connect System offers the same superior recording quality of Rhythmlink’s original MR Conditional/CT electrodes, allowing electrodes to remain on the patient while in a 1.5T and 3T MR scanner. Once imaging is complete, patients are quickly and efficiently reconnected to their monitoring device via Rhythmlink’s new Quick Connect System, resulting in both time and costs saved and improved patient care.
“We are extremely proud to be releasing the new Quick Connect System,” said Shawn Regan, CEO of Rhythmlink International. “Since releasing the first FDA Cleared MR Conditional electrodes several years ago, our team has been working hard to improve the product, and to reach the next step in efficient patient care. We believe the Quick Connect System will meet a need in healthcare that many have been waiting for.”
Each new product number features numbered heat shrink and flags designed to assist bedside personnel such as nursing teams to easily disconnect and reconnect patients without removing the EEG electrodes when the patient requires a CT or magnetic resonance imaging (MRI). Electrodes are available in Slim Cup, a Deep Cup design that holds more gel, and a Webb design that provides a larger recording surface. All three designs can be mixed to help reduce skin breakdown and increase patient comfort, and custom array options will be available in the future to meet additional patient needs.
“These products offer the next level of high efficiency workflow,” Vice President of Global Sales Leah Hanson, R.EEG/EP T., said. “The Quick Connect System not only eliminates the need for removal and reapplication of EEG electrodes when a patient needs a CT or MRI, but reduces the overall time of the disconnection and reconnection process. It demonstrates that at Rhythmlink we understand that every moment counts when it comes to patient care.”
The new Quick Connect System adds to their extensive product lines focused on brain health, routine EEG monitoring, ICU, and more.
Rhythmlink International is a medical device manufacturing company specializing in devices that help connect patients to machines to record or elicit physiologic information. Rhythmlink designs, manufactures and distributes a variety of medical devices for intraoperative neuromonitoring, electroencephalography, evoked potentials, polysomnography, long-term monitoring epilepsy and critical care units. Founded by neurodiagnostic technicians and engineers in 2002, Rhythmlink also offers custom packaging, custom products, private labeling and contract manufacturing services.