Business Wire12.11.17
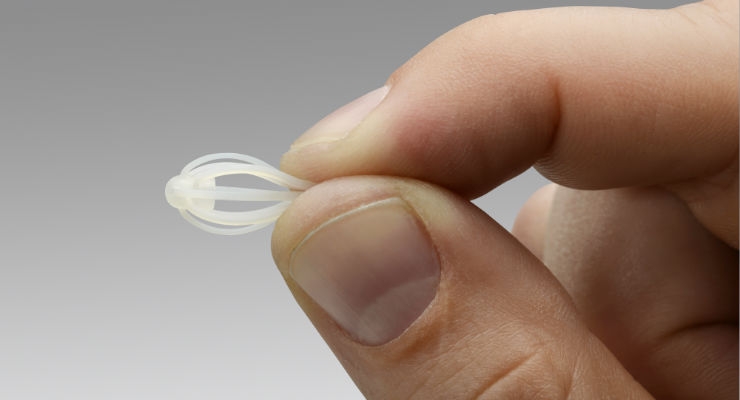
Intersect ENT Inc., a company dedicated to transforming care for patients with ear, nose and throat conditions, announced that it has received approval from the U.S. Food and Drug Administration (FDA) for the SINUVA (mometasone furoate) Sinus Implant, a new targeted approach to treating recurrent nasal polyp disease in patients who have had previous ethmoid sinus surgery.
Placed during a routine physician office visit, SINUVA expands into the sinus cavity and delivers an anti-inflammatory steroid directly to the site of polyp disease for 90 days. Results from a randomized clinical trial demonstrated a 63 percent relative reduction in bilateral polyp grade (a measurement of the extent of ethmoid polyp disease) for patients treated with SINUVA, compared to control.
“SINUVA represents a much-needed breakthrough for the many nasal polyp sufferers who are seeking an effective treatment,” said Robert C. Kern, M.D., Chairman of Otolaryngology—Head and Neck Surgery at Northwestern University Feinberg School of Medicine, who served as national co-principal investigator of the pivotal study of the implant. “For many patients struggling to manage this disease, the current treatment approaches of repeat surgeries and high-dose oral steroids have significant limitations, and intranasal sprays and rinses rely heavily on patient compliance. I look forward to offering SINUVA to my patients.”
Nasal polyps are inflammatory growths along the lining of nasal passages or sinuses that can cause nasal congestion, infections and loss of sense of smell. Many people with chronic sinusitis and nasal polyps return to their ENT specialist with symptoms within the first year following initial treatment. Approximately 635,000 Americans have had previous sinus surgery and continue to see their ENT physicians for treatment of recurring symptoms.
“For more than a decade Intersect ENT has been focused on developing innovative therapies for chronic sinusitis sufferers. We are pleased that the approval of SINUVA will give patients with recurrent nasal polyps a new option,” said Lisa Earnhardt, president and CEO of Intersect ENT. “This FDA approval—our fourth commercial product, and our first product to be regulated as a pharmaceutical—is an exciting milestone for our team. We look forward to introducing SINUVA to physicians across the country in the coming months as we work toward our second-quarter launch.”
The FDA submission for the SINUVA Implant was supported by the results of clinical studies of 400 patients, including the landmark RESOLVE II pivotal study. RESOLVE II met its co-primary efficacy endpoints, which included a statistically significant reduction from baseline in bilateral polyp grade (p=0.007) and a reduction from baseline Nasal Obstruction/Congestion score (p=0.007). Secondary endpoints achieving statistical significance through day 90 include the proportion of patients still indicated for repeat sinus surgery and improvements in sense of smell, sense of nasal congestion and percent ethmoid sinus obstruction.
The FDA did not require any post-approval clinical trials.
Placed during a routine physician office visit, SINUVA expands into the sinus cavity and delivers an anti-inflammatory steroid directly to the site of polyp disease for 90 days. Results from a randomized clinical trial demonstrated a 63 percent relative reduction in bilateral polyp grade (a measurement of the extent of ethmoid polyp disease) for patients treated with SINUVA, compared to control.
“SINUVA represents a much-needed breakthrough for the many nasal polyp sufferers who are seeking an effective treatment,” said Robert C. Kern, M.D., Chairman of Otolaryngology—Head and Neck Surgery at Northwestern University Feinberg School of Medicine, who served as national co-principal investigator of the pivotal study of the implant. “For many patients struggling to manage this disease, the current treatment approaches of repeat surgeries and high-dose oral steroids have significant limitations, and intranasal sprays and rinses rely heavily on patient compliance. I look forward to offering SINUVA to my patients.”
Nasal polyps are inflammatory growths along the lining of nasal passages or sinuses that can cause nasal congestion, infections and loss of sense of smell. Many people with chronic sinusitis and nasal polyps return to their ENT specialist with symptoms within the first year following initial treatment. Approximately 635,000 Americans have had previous sinus surgery and continue to see their ENT physicians for treatment of recurring symptoms.
“For more than a decade Intersect ENT has been focused on developing innovative therapies for chronic sinusitis sufferers. We are pleased that the approval of SINUVA will give patients with recurrent nasal polyps a new option,” said Lisa Earnhardt, president and CEO of Intersect ENT. “This FDA approval—our fourth commercial product, and our first product to be regulated as a pharmaceutical—is an exciting milestone for our team. We look forward to introducing SINUVA to physicians across the country in the coming months as we work toward our second-quarter launch.”
The FDA submission for the SINUVA Implant was supported by the results of clinical studies of 400 patients, including the landmark RESOLVE II pivotal study. RESOLVE II met its co-primary efficacy endpoints, which included a statistically significant reduction from baseline in bilateral polyp grade (p=0.007) and a reduction from baseline Nasal Obstruction/Congestion score (p=0.007). Secondary endpoints achieving statistical significance through day 90 include the proportion of patients still indicated for repeat sinus surgery and improvements in sense of smell, sense of nasal congestion and percent ethmoid sinus obstruction.
The FDA did not require any post-approval clinical trials.