Business Wire11.13.17
Neural Analytics Inc., a medical device company developing and commercializing technology to measure, diagnose, and track brain health, today announced the results from the first phase of the EXPEDITE (Expedite a NeXt Generation PortablE Diagnostic Platform for Determination and Immediate Triage of Emergency Large Vessel Stroke) program, which (under IRB approved investigation) demonstrates the company’s advanced Transcranial Doppler (TCD) technology platform was accurate with over 94 percent area under the curve (AUC) for early measurement of acute ischemic stroke (AIS) due to large vessel occlusion (LVO). The data was presented at the 10th annual Society of Vascular and Interventional Neurology (SVIN) meeting in Boston, MA.
“The research results of the EXPEDITE program demonstrate that Neural Analytics’ portable diagnostic platform, the Lucid System, has the potential to influence how fast we detect stroke, triage and treat patients,” said Thomas Devlin, M.D., medical director of Erlanger’s Southeast Regional Stroke Center, 2017 SVIN Stroke Workshop chair and principal investigator of the study. “We are honored to be the first site in the world to evaluate this technology with the goal of improving our stroke patient outcomes.”
Dr. Devlin presented the data demonstrating that Neural Analytics’ Lucid System is capable of measuring and displaying LVO with 91 percent sensitivity and 85 percent specificity as compared to the current standard of care imaging in persons suspected of having a stroke.
The first phase of the EXPEDITE program enrolled patients at Erlanger Southeast Regional Stroke Center in Chattanooga, Tenn., and Baptist Stroke & Cerebrovascular Center in Jacksonville, Fla., to examine the feasibility of Neural Analytics’ Lucid TCD Ultrasound System in the measurement of cerebral blood flow in patients with LVO.
“With the Lucid System, we can look inside the brain at blood flow patterns in real time and identify physiological changes associated with brain disorders including stroke,” said Robert Hamilton, Ph.D., co-founder and chief scientific officer of Neural Analytics. “This technology has the potential to dramatically influence how we measure and track patients with serious brain injuries all across the world.”
The Lucid System studied in the EXPEDITE program is comprised of two key components: an FDA cleared transcranial ultrasound system, and an IRB-approved investigational automation technology designed to simplify data collection for healthcare practitioners. Advanced technologies like the Lucid System can dramatically improve healthcare management for patients who suffer from stroke each year by providing a device to help physicians triage these patients quickly and accurately.
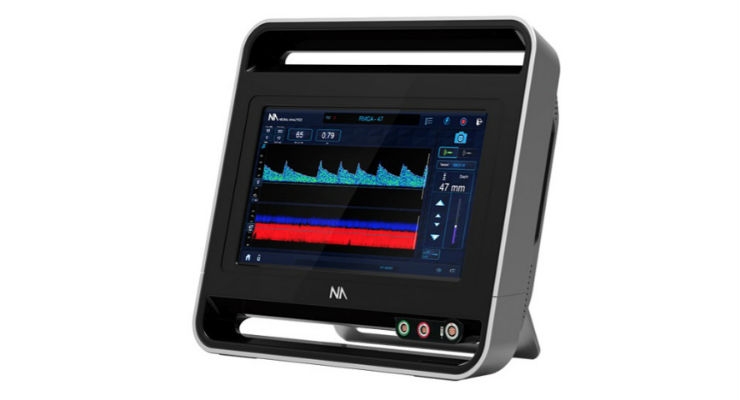
The Lucid System uses TCD ultrasound to assess the brain’s blood vessels from outside the body. This analysis is non-invasive, can be performed in the physician’s office, and helps the physician diagnose brain disorders, potentially without the need for additional, more invasive tests. Many significant brain disorders, such as LVO, cause dangerous blood flow disruptions. The Lucid System is a battery-operated, medical-grade tablet device designed to be transported easily throughout a medical facility and in a range of settings that require the rapid assessment of blood flow in the brain to expedite treatment.
The research study evaluated 107 subjects either experiencing computed tomography angiography (CTA)-confirmed AIS due to LVO of the internal carotid artery (ICA) or middle cerebral artery (MCA) or healthy controls. TCD scans in LVO patients were recorded in 30 second intervals across multiple depths for each brain hemisphere while patients were treated with a drug or awaited endovascular or surgical treatment.
Globally, and each year, strokes affect about 16 million people and kill an estimated 5.7 million people with an annual U.S. healthcare cost of $104 billion.1-4 Stroke is a time sensitive disease and requires intervention as soon as possible. Without appropriate diagnosis and treatment of stroke, a majority of patients may suffer permanent disability or death. Despite recent advances in life saving surgical treatments, less than 10 percent of eligible stroke patients are treated surgically due to the lack of a portable diagnostic device for early detection.2-4
Neural Analytics’ Lucid M1 System is FDA cleared and CE marked for the monitoring of blood flow velocities in the cerebral vasculature.
References
1 Ganesalingam, J. Cost Utility Analysis of Mechanical Thrombectomy Using Stent Retrievers in Acute Ischemic Stroke. Stroke. 2015 Sep;46(9):2591-8. doi: 10.1161/STROKEAHA.115.009396. Epub 2015 Aug 6.
2 Saver, J. et. Al. Stent Retriever Thrombectomy after Intraveneous t-PA vs. t_PA alone in stroke. N Engl J Med 2015; 372:2285-2295.
3 Ovbiagele, B. et. Al Forecasting the Future of Stroke in the United States. Stroke. 2013. http://stroke.ahajournals.org/content/44/8/2361.abstract.
4 Age Ageing (2009) 38 (1): 4-5.doi: 10.1093/ageing/afn282.
“The research results of the EXPEDITE program demonstrate that Neural Analytics’ portable diagnostic platform, the Lucid System, has the potential to influence how fast we detect stroke, triage and treat patients,” said Thomas Devlin, M.D., medical director of Erlanger’s Southeast Regional Stroke Center, 2017 SVIN Stroke Workshop chair and principal investigator of the study. “We are honored to be the first site in the world to evaluate this technology with the goal of improving our stroke patient outcomes.”
Dr. Devlin presented the data demonstrating that Neural Analytics’ Lucid System is capable of measuring and displaying LVO with 91 percent sensitivity and 85 percent specificity as compared to the current standard of care imaging in persons suspected of having a stroke.
The first phase of the EXPEDITE program enrolled patients at Erlanger Southeast Regional Stroke Center in Chattanooga, Tenn., and Baptist Stroke & Cerebrovascular Center in Jacksonville, Fla., to examine the feasibility of Neural Analytics’ Lucid TCD Ultrasound System in the measurement of cerebral blood flow in patients with LVO.
“With the Lucid System, we can look inside the brain at blood flow patterns in real time and identify physiological changes associated with brain disorders including stroke,” said Robert Hamilton, Ph.D., co-founder and chief scientific officer of Neural Analytics. “This technology has the potential to dramatically influence how we measure and track patients with serious brain injuries all across the world.”
The Lucid System studied in the EXPEDITE program is comprised of two key components: an FDA cleared transcranial ultrasound system, and an IRB-approved investigational automation technology designed to simplify data collection for healthcare practitioners. Advanced technologies like the Lucid System can dramatically improve healthcare management for patients who suffer from stroke each year by providing a device to help physicians triage these patients quickly and accurately.
The Lucid System uses TCD ultrasound to assess the brain’s blood vessels from outside the body. This analysis is non-invasive, can be performed in the physician’s office, and helps the physician diagnose brain disorders, potentially without the need for additional, more invasive tests. Many significant brain disorders, such as LVO, cause dangerous blood flow disruptions. The Lucid System is a battery-operated, medical-grade tablet device designed to be transported easily throughout a medical facility and in a range of settings that require the rapid assessment of blood flow in the brain to expedite treatment.
The research study evaluated 107 subjects either experiencing computed tomography angiography (CTA)-confirmed AIS due to LVO of the internal carotid artery (ICA) or middle cerebral artery (MCA) or healthy controls. TCD scans in LVO patients were recorded in 30 second intervals across multiple depths for each brain hemisphere while patients were treated with a drug or awaited endovascular or surgical treatment.
Globally, and each year, strokes affect about 16 million people and kill an estimated 5.7 million people with an annual U.S. healthcare cost of $104 billion.1-4 Stroke is a time sensitive disease and requires intervention as soon as possible. Without appropriate diagnosis and treatment of stroke, a majority of patients may suffer permanent disability or death. Despite recent advances in life saving surgical treatments, less than 10 percent of eligible stroke patients are treated surgically due to the lack of a portable diagnostic device for early detection.2-4
Neural Analytics’ Lucid M1 System is FDA cleared and CE marked for the monitoring of blood flow velocities in the cerebral vasculature.
References
1 Ganesalingam, J. Cost Utility Analysis of Mechanical Thrombectomy Using Stent Retrievers in Acute Ischemic Stroke. Stroke. 2015 Sep;46(9):2591-8. doi: 10.1161/STROKEAHA.115.009396. Epub 2015 Aug 6.
2 Saver, J. et. Al. Stent Retriever Thrombectomy after Intraveneous t-PA vs. t_PA alone in stroke. N Engl J Med 2015; 372:2285-2295.
3 Ovbiagele, B. et. Al Forecasting the Future of Stroke in the United States. Stroke. 2013. http://stroke.ahajournals.org/content/44/8/2361.abstract.
4 Age Ageing (2009) 38 (1): 4-5.doi: 10.1093/ageing/afn282.