Business Wire11.09.17
CorInnova Inc., an emerging medical device company developing heart failure treatments, has received notice of allowance of a seminal patent to protect its intellectual property associated with the world’s first minimally invasively-delivered soft robotic heart device to support heart function. The company also has become a resident at Johnson & Johnson Innovation, JLABS @ TMC following a $6.1 million funding from the Wellcome Trust, details of which have not been disclosed.
“Our EpicHeartTM technology is innovative and groundbreaking. We believe it will lead to a new paradigm for heart failure treatment,” said William Altman, CEO of CorInnova.
The technology includes the following features:
CorInnova’s operations are located at Johnson & Johnson Innovation, JLABS at the Texas Medical Center (JLABS @ TMC). JLABS is a 34,000 square-foot life science innovation center located in Houston. The labs provide a flexible environment for start-up companies pursuing new technologies and research platforms to advance medical care. Through a "no strings attached" model, JJI does not take an equity stake in the companies occupying JLABS, and the companies are free to develop products—either on their own, or by initiating a separate external partnership with JJI or any other company.
“Wellcome is pleased to support the development of this innovative technology for the treatment of congestive heart failure,” said Dr. Philip Jordan from Wellcome’s Innovations team. “Congestive heart failure is a chronic condition that affects roughly six million people in the U.S. alone and is a leading cause of death and disability. A significantly less-invasive medical device that could restore a certain level of heart function would be potentially transformative for patients.”
Watch the video below to learn how the CorInnova EpicHeart Soft Robotic Heart Assist Device is delivered minimally invasively:
“We are honored to have our work recognized by the Wellcome Trust. We are further honored to have been chosen by JLABS as one of a limited number of companies to be invited to join its life sciences incubator,” added Altman. “Due to its minimally invasive nature and its potential for reduced adverse events from blood contact, CorInnova’s non-blood-contacting soft robotic cardiac assist device could potentially triple or quadruple the number of heart failure patients who could be eligible for such a life-saving device therapy.”
Further validation of the EpicHeart technology occurred in September, when CorInnova was the winner of a $50,000 award at the Fifth Annual Pediatric Device Innovation Symposium organized by the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Health System and funded by the U.S. Food and Drug Administration National Capital Consortium for Pediatric Device Innovation. This competition is designed to foster innovation that will advance pediatric healthcare and address unmet surgical and medical needs for children.
“We are pleased that the compelling advantages of CorInnova’s technology for heart failure treatment in adults is equally compelling for treatment for children,” said Boris Leschinsky, CorInnova’s vice president of Product Development.
Heart failure is a condition in which the heart is unable to supply sufficient blood flow to meet the body’s needs. There is no cure. About 6 million people in the Untied States have heart failure. The 1 million U.S. and European Union patients in end-stage congestive heart failure have an extremely poor prognosis (40 percent two-year mortality). Transplant is the preferred treatment, but only 4,500 hearts are available worldwide. Increasingly, advanced left ventricular assist devices (LVADs) are used to prolong life, but their utilization is limited by invasive surgery, invasive attachment to the heart and aorta, and blood contact, leading to adverse events such as blood clots and stroke, as well as bleeding from necessary anti-coagulant therapy and blood damage. Only about 7,000 to 8,000 LVADs are implanted annually.
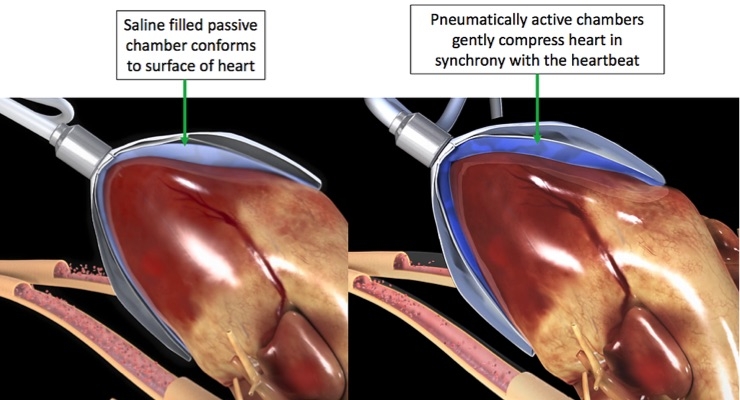
CorInnova has developed a direct cardiac compression device whose technology is a significant break with the prior art. CorInnova’s biventricular device is a collapsible thin-film pneumatically actuated soft robotic device that surrounds both ventricles of the heart. Air inflates the device in synchrony with the heart and increases cardiac output by gently squeezing the heart. CorInnova has also developed a collapsible self-expanding device design that simplifies and speeds implantation. Due to the minimally invasive technology, hospital stays could potentially be reduced from 30 days to four to six days, compared to LVADs, and overall adverse events compared to LVADs could be reduced up to 30 percent to 40 percent. CorInnova’s device can potentially be used for a range of end-stage heart failure patients for cardiac assist, ranging from short-term “bridge to decision” use, medium-term “bridge to transplant” use, and all the way to permanent “destination therapy” use. Diastolic as well as systolic heart failure patients may benefit from the technology. Diastolic heart failure patients currently have no approved device treatment.
CorInnova is a medical device company founded to commercialize technology developed by the Cardiac Mechanics Laboratory of Dr. John C. Criscione, professor in the Department of Biomedical Engineering at Texas A&M University. The company has been supported by a group of private investors, federal institutions including the NIH National Heart Lung Blood Institute (NHLBI) and National Science Foundation (NSF), and the Texas Emerging Technology Fund. The company is headquartered in Houston, Texas.
Wellcome is a global charitable foundation that is both politically and financially independent. Wellcome supports scientists and researchers.
“Our EpicHeartTM technology is innovative and groundbreaking. We believe it will lead to a new paradigm for heart failure treatment,” said William Altman, CEO of CorInnova.
The technology includes the following features:
- First collapsible and self-expanding thin film soft robotic device for cardiac assist;
- Rapid minimally invasive implantation ability and simple delivery tool;
- Intrinsic pneumatic attachment inside the pericardial sac (no sutures or incisions to the heart or aorta);
- Non-blood-contacting device operation;
- Likelihood of up to 30 percent to 40 percent fewer adverse events than blood-contacting assist devices;
- Biventricular (or normal left ventricular) assist capability;
- Non-obligatory operation;
- Promotion of heart rehabilitation by promoting correct cardiac motion;
- The potential to prevent the development of heart failure after major heart attacks.
CorInnova’s operations are located at Johnson & Johnson Innovation, JLABS at the Texas Medical Center (JLABS @ TMC). JLABS is a 34,000 square-foot life science innovation center located in Houston. The labs provide a flexible environment for start-up companies pursuing new technologies and research platforms to advance medical care. Through a "no strings attached" model, JJI does not take an equity stake in the companies occupying JLABS, and the companies are free to develop products—either on their own, or by initiating a separate external partnership with JJI or any other company.
“Wellcome is pleased to support the development of this innovative technology for the treatment of congestive heart failure,” said Dr. Philip Jordan from Wellcome’s Innovations team. “Congestive heart failure is a chronic condition that affects roughly six million people in the U.S. alone and is a leading cause of death and disability. A significantly less-invasive medical device that could restore a certain level of heart function would be potentially transformative for patients.”
Watch the video below to learn how the CorInnova EpicHeart Soft Robotic Heart Assist Device is delivered minimally invasively:
“We are honored to have our work recognized by the Wellcome Trust. We are further honored to have been chosen by JLABS as one of a limited number of companies to be invited to join its life sciences incubator,” added Altman. “Due to its minimally invasive nature and its potential for reduced adverse events from blood contact, CorInnova’s non-blood-contacting soft robotic cardiac assist device could potentially triple or quadruple the number of heart failure patients who could be eligible for such a life-saving device therapy.”
Further validation of the EpicHeart technology occurred in September, when CorInnova was the winner of a $50,000 award at the Fifth Annual Pediatric Device Innovation Symposium organized by the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Health System and funded by the U.S. Food and Drug Administration National Capital Consortium for Pediatric Device Innovation. This competition is designed to foster innovation that will advance pediatric healthcare and address unmet surgical and medical needs for children.
“We are pleased that the compelling advantages of CorInnova’s technology for heart failure treatment in adults is equally compelling for treatment for children,” said Boris Leschinsky, CorInnova’s vice president of Product Development.
Heart failure is a condition in which the heart is unable to supply sufficient blood flow to meet the body’s needs. There is no cure. About 6 million people in the Untied States have heart failure. The 1 million U.S. and European Union patients in end-stage congestive heart failure have an extremely poor prognosis (40 percent two-year mortality). Transplant is the preferred treatment, but only 4,500 hearts are available worldwide. Increasingly, advanced left ventricular assist devices (LVADs) are used to prolong life, but their utilization is limited by invasive surgery, invasive attachment to the heart and aorta, and blood contact, leading to adverse events such as blood clots and stroke, as well as bleeding from necessary anti-coagulant therapy and blood damage. Only about 7,000 to 8,000 LVADs are implanted annually.
CorInnova has developed a direct cardiac compression device whose technology is a significant break with the prior art. CorInnova’s biventricular device is a collapsible thin-film pneumatically actuated soft robotic device that surrounds both ventricles of the heart. Air inflates the device in synchrony with the heart and increases cardiac output by gently squeezing the heart. CorInnova has also developed a collapsible self-expanding device design that simplifies and speeds implantation. Due to the minimally invasive technology, hospital stays could potentially be reduced from 30 days to four to six days, compared to LVADs, and overall adverse events compared to LVADs could be reduced up to 30 percent to 40 percent. CorInnova’s device can potentially be used for a range of end-stage heart failure patients for cardiac assist, ranging from short-term “bridge to decision” use, medium-term “bridge to transplant” use, and all the way to permanent “destination therapy” use. Diastolic as well as systolic heart failure patients may benefit from the technology. Diastolic heart failure patients currently have no approved device treatment.
CorInnova is a medical device company founded to commercialize technology developed by the Cardiac Mechanics Laboratory of Dr. John C. Criscione, professor in the Department of Biomedical Engineering at Texas A&M University. The company has been supported by a group of private investors, federal institutions including the NIH National Heart Lung Blood Institute (NHLBI) and National Science Foundation (NSF), and the Texas Emerging Technology Fund. The company is headquartered in Houston, Texas.
Wellcome is a global charitable foundation that is both politically and financially independent. Wellcome supports scientists and researchers.