Abbott Laboratories10.12.17
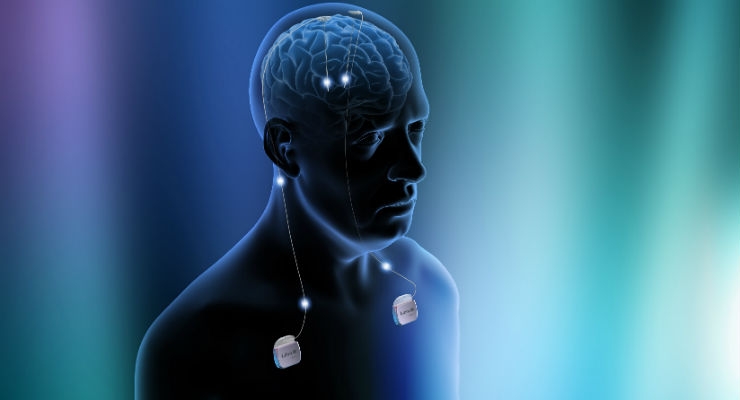
New data published in The Lancet: Psychiatry has found that deep brain stimulation (DBS) may offer some patients an option for managing their chronic, treatment-resistant depression. The data, which stems from the Abbott-sponsored BROADEN Study, also provides safety and feasibility results of DBS therapy as a treatment for these patients and the authors concluded that after 24 months of stimulation, nearly half of all DBS patients responded to the therapy. Of these patients, 26 percent of patients experienced remission of their depression; a remission rate that steadily grew over time.
While the BROADEN study initially found no statistically significant difference in efficacy between the stimulation group and the control group after six and 12 months, after the initial 12-month study, 77 of 90 participants entered into a four-year follow-up study. Within that follow-up study, the authors found that patients receiving DBS therapy saw response and remission rates of 29 percent and 14 percent at 12 months, 53 percent and 18 percent at 18 months, and 49 percent and 26 percent at 24 months, respectively. Currently, DBS is not currently indicated for depression and Abbott is FDA-approved only to offer DBS for essential tremor and Parkinson's disease.
"Innovation within the field of neuroscience takes time and is filled with opportunities to learn, adapt and learn again. This study is a strong example of how our therapies can contribute to the innovation taking place within the broad field of neuroscience," said Allen Burton, M.D. medical director within Abbott's Neuromodulation Division. "We applaud the researchers who led this study and look forward to future advancements to support the care of people suffering from chronic, treatment-resistant depression."
Depression affects more than 21 million adults in the U.S., according to the National Institute of Mental Health. Nearly 4 million people live with severe depression that doesn’t respond to traditional treatment or multiple treatment attempts.
"While I am disappointed by the initial results, I’m encouraged by the long-term outcomes seen in this trial, which are consistent with previous and ongoing experience with DBS outside of this clinical trial," said Helen Mayberg, M.D., professor of psychiatry, neurology and radiology at Emory University in Atlanta, Ga. "There are refinements to optimize DBS delivery that may prove useful to understand these findings and move the therapy forward. For example, we now know that implantation method and directionality matter for optimal patient outcomes. We look forward to seeing what new innovations, such as use of advanced imaging to guide the implantation and use of directional leads, can do in the future."
Assessing a New Approach to Depression Treatment
Clinical research has often implicated activity within an area of the brain known as “Brodmann Area 25” as compounding treatment-resistant depression. Researchers have pointed to DBS as a new option for patients because modern systems can precisely target stimulation to this area and deliver remission to patients who had not responded to prior therapy attempts.
Abbott originally launched the randomized controlled BROADEN study in 2008 to assess DBS therapy in patients with chronic, treatment-resistant depression. The study, which utilized the investigational Abbott Libra deep brain stimulation system, built upon a pilot study that showed meaningful reductions in depression in patients receiving DBS therapy and on the groundbreaking work of Dr. Mayberg and colleagues that supported the application of DBS therapy in patients with treatment-resistant depression. When the BROADEN study was discontinued, patients were then monitored in a follow-up study.
The BROADEN study enrolled 128 patients and implanted 90 between the ages of 21 and 70 years of age at 13 centers who had been diagnosed with major depressive disorder. Patients must have tried at least four treatments for their depression without a meaningful treatment response. Patients should talk to their physician about the benefits and risks of any DBS therapy option and should consider this data as investigational in nature and not indicative.
While the BROADEN study initially found no statistically significant difference in efficacy between the stimulation group and the control group after six and 12 months, after the initial 12-month study, 77 of 90 participants entered into a four-year follow-up study. Within that follow-up study, the authors found that patients receiving DBS therapy saw response and remission rates of 29 percent and 14 percent at 12 months, 53 percent and 18 percent at 18 months, and 49 percent and 26 percent at 24 months, respectively. Currently, DBS is not currently indicated for depression and Abbott is FDA-approved only to offer DBS for essential tremor and Parkinson's disease.
"Innovation within the field of neuroscience takes time and is filled with opportunities to learn, adapt and learn again. This study is a strong example of how our therapies can contribute to the innovation taking place within the broad field of neuroscience," said Allen Burton, M.D. medical director within Abbott's Neuromodulation Division. "We applaud the researchers who led this study and look forward to future advancements to support the care of people suffering from chronic, treatment-resistant depression."
Depression affects more than 21 million adults in the U.S., according to the National Institute of Mental Health. Nearly 4 million people live with severe depression that doesn’t respond to traditional treatment or multiple treatment attempts.
"While I am disappointed by the initial results, I’m encouraged by the long-term outcomes seen in this trial, which are consistent with previous and ongoing experience with DBS outside of this clinical trial," said Helen Mayberg, M.D., professor of psychiatry, neurology and radiology at Emory University in Atlanta, Ga. "There are refinements to optimize DBS delivery that may prove useful to understand these findings and move the therapy forward. For example, we now know that implantation method and directionality matter for optimal patient outcomes. We look forward to seeing what new innovations, such as use of advanced imaging to guide the implantation and use of directional leads, can do in the future."
Assessing a New Approach to Depression Treatment
Clinical research has often implicated activity within an area of the brain known as “Brodmann Area 25” as compounding treatment-resistant depression. Researchers have pointed to DBS as a new option for patients because modern systems can precisely target stimulation to this area and deliver remission to patients who had not responded to prior therapy attempts.
Abbott originally launched the randomized controlled BROADEN study in 2008 to assess DBS therapy in patients with chronic, treatment-resistant depression. The study, which utilized the investigational Abbott Libra deep brain stimulation system, built upon a pilot study that showed meaningful reductions in depression in patients receiving DBS therapy and on the groundbreaking work of Dr. Mayberg and colleagues that supported the application of DBS therapy in patients with treatment-resistant depression. When the BROADEN study was discontinued, patients were then monitored in a follow-up study.
The BROADEN study enrolled 128 patients and implanted 90 between the ages of 21 and 70 years of age at 13 centers who had been diagnosed with major depressive disorder. Patients must have tried at least four treatments for their depression without a meaningful treatment response. Patients should talk to their physician about the benefits and risks of any DBS therapy option and should consider this data as investigational in nature and not indicative.