Business Wire10.06.17
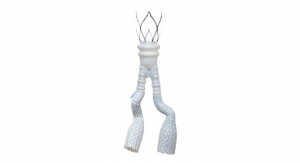
Endologix Inc., a developer and marketer of treatments for aortic disorders, has received CE Mark approval for its Nellix EndoVascular Aneurysm Sealing System (Nellix) with the refined Indications for Use (IFU). Nellix is being studied in the United States under an Investigational Device Exemption (IDE).
Following a thorough review of supporting clinical data, the company’s notified body, together with an independent clinical reviewer, has determined that Nellix, with the refined IFU, meets the applicable safety and clinical performance requirements. As a result of these evaluations, the notified body has granted a CE Mark for Nellix with the refined IFU.
“We are very pleased with the clinical outcomes generated by the Nellix EndoVascular Aneurysm Sealing System utilizing the refined IFU,” commented John McDermott, Endologix’s CEO. “The Nellix CE Mark with the refined IFU provides patients and physicians in Europe with continued access to the clinical benefits of complete aneurysm sealing, including low rates of endoleaks and all-cause mortality.”1
Endologix also said it has received IDE approval from the U.S. Food and Drug Administration (FDA) to begin a confirmatory clinical study to evaluate the safety and effectiveness of the Nellix EndoVascular Aneurysm Sealing System (EVAS) for the endovascular treatment of infrarenal abdominal aortic aneurysms.
The EVAS2 IDE Multicenter Safety and Effectiveness Confirmatory Study (EVAS2) will prospectively evaluate the refined IFU and the Nellix Gen2 EVAS System. The study is approved to enroll up to 90 primary patients, with one-year follow-up data required for the pre-market approval application (PMA).
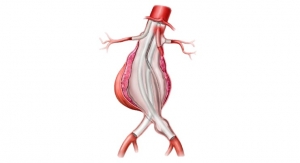
The Nellix EVAS system is an endovascular abdominal aortic aneurysm (AAA) therapy designed to seal the entire aneurysm Nellix is the first and only EVAS product developed as an alternative treatment approach to traditional EVAR devices.
"We are pleased to receive IDE approval from the FDA to begin this confirmatory study and look forward to collaborating with the investigators to achieve the goal of commencing enrollment by the end of this year," McDermott said. "Based on the anticipated enrollment timeline, one-year follow up period, and regulatory review process, we continue to estimate PMA approval in 2020."
Endologix Inc. develops and manufactures minimally invasive treatments for aortic disorders. The Irvine, Calif.-based company's focus is endovascular stent grafts for the treatment of abdominal aortic aneurysms. AAA is a weakening of the wall of the aorta, the largest artery in the body, resulting in a balloon-like enlargement. Once AAA develops, it continues to enlarge and, if left untreated, becomes increasingly susceptible to rupture. The overall patient mortality rate for ruptured AAA is approximately 80 percent, making it a leading cause of death in the United States.
Reference
1. Carpenter, J.P. (2017, June). Nellix System for Endovascular Aneurysm Sealing: Effect of IFU Refinements on Outcomes from the EVAS FORWARD IDE. Presented at the 2017 Vascular Annual Meeting, San Diego, CA.
Following a thorough review of supporting clinical data, the company’s notified body, together with an independent clinical reviewer, has determined that Nellix, with the refined IFU, meets the applicable safety and clinical performance requirements. As a result of these evaluations, the notified body has granted a CE Mark for Nellix with the refined IFU.
“We are very pleased with the clinical outcomes generated by the Nellix EndoVascular Aneurysm Sealing System utilizing the refined IFU,” commented John McDermott, Endologix’s CEO. “The Nellix CE Mark with the refined IFU provides patients and physicians in Europe with continued access to the clinical benefits of complete aneurysm sealing, including low rates of endoleaks and all-cause mortality.”1
Endologix also said it has received IDE approval from the U.S. Food and Drug Administration (FDA) to begin a confirmatory clinical study to evaluate the safety and effectiveness of the Nellix EndoVascular Aneurysm Sealing System (EVAS) for the endovascular treatment of infrarenal abdominal aortic aneurysms.
The EVAS2 IDE Multicenter Safety and Effectiveness Confirmatory Study (EVAS2) will prospectively evaluate the refined IFU and the Nellix Gen2 EVAS System. The study is approved to enroll up to 90 primary patients, with one-year follow-up data required for the pre-market approval application (PMA).
The Nellix EVAS system is an endovascular abdominal aortic aneurysm (AAA) therapy designed to seal the entire aneurysm Nellix is the first and only EVAS product developed as an alternative treatment approach to traditional EVAR devices.
"We are pleased to receive IDE approval from the FDA to begin this confirmatory study and look forward to collaborating with the investigators to achieve the goal of commencing enrollment by the end of this year," McDermott said. "Based on the anticipated enrollment timeline, one-year follow up period, and regulatory review process, we continue to estimate PMA approval in 2020."
Endologix Inc. develops and manufactures minimally invasive treatments for aortic disorders. The Irvine, Calif.-based company's focus is endovascular stent grafts for the treatment of abdominal aortic aneurysms. AAA is a weakening of the wall of the aorta, the largest artery in the body, resulting in a balloon-like enlargement. Once AAA develops, it continues to enlarge and, if left untreated, becomes increasingly susceptible to rupture. The overall patient mortality rate for ruptured AAA is approximately 80 percent, making it a leading cause of death in the United States.
Reference
1. Carpenter, J.P. (2017, June). Nellix System for Endovascular Aneurysm Sealing: Effect of IFU Refinements on Outcomes from the EVAS FORWARD IDE. Presented at the 2017 Vascular Annual Meeting, San Diego, CA.