PR Newswire10.02.17
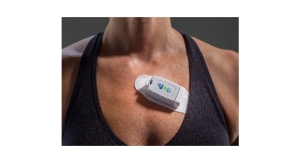
Qualcomm Incorporated, through its wholly owned subsidiary, Qualcomm Life Inc., announced that it has designed and developed breakthrough reference designs for cost-effective, connected and medical-grade biometric patches that will enable a multitude of intelligent care models, from perioperative care to assessing the impact of therapeutic interventions. Designed to allow health care professionals to monitor patients across the care continuum with access to near real-time data, the patches measure a variety of biometric parameters, including clinical thermometry and sophisticated motion measurements. Benchmark Electronics Inc., a global provider of integrated electronics manufacturing, design and engineering services, will license the reference designs and serve as the device design and manufacturer of record with the FDA for those sensors.
"This wearable patch technology will be transformative in its ability to provide timely and accurate data to enable care providers to make better-informed decisions," said James Mault, MD, FACS, senior vice president and chief medical officer, Qualcomm Life. "Utilizing Qualcomm's 30 years of leadership in inventing new connected "things," this low-power, cost-effective, single-use design will fuel new, scalable care models as we transition as an industry from episodic, reactive care to more continuous, proactive, intelligent care."
As a part of the collaboration, Qualcomm Incorporated, Qualcomm Life Inc., and Benchmark Electronics entered into a Healthcare Product License Agreement, which allows Benchmark to license reference designs for Qualcomm Life's biometric patches. Clinical validation is currently underway, and the patches are slated to be commercially available through Benchmark in 2018.
"We are thrilled to work with Qualcomm Life, a global leader in inventing revolutionary connected technologies, to create a new packaged sensor offering for health care companies that is economical, secure and user friendly," said Paul Tufano, CEO, Benchmark Electronics. "This collaboration aligns with our mission to deliver high-value solutions and innovative technology for our customers."
The innovative, cost-effective patches were developed on Qualcomm Life's 2net Design platform, a reference design platform for electronics modules which power connected medical devices, including disposable drug delivery devices and disposable diagnostic devices. 2net Design leverages Qualcomm's expertise in developing innovative reference designs for wireless, power-efficient, small-integrated modules to create smarter, more affordable and intuitive medical devices.
"This wearable patch technology will be transformative in its ability to provide timely and accurate data to enable care providers to make better-informed decisions," said James Mault, MD, FACS, senior vice president and chief medical officer, Qualcomm Life. "Utilizing Qualcomm's 30 years of leadership in inventing new connected "things," this low-power, cost-effective, single-use design will fuel new, scalable care models as we transition as an industry from episodic, reactive care to more continuous, proactive, intelligent care."
As a part of the collaboration, Qualcomm Incorporated, Qualcomm Life Inc., and Benchmark Electronics entered into a Healthcare Product License Agreement, which allows Benchmark to license reference designs for Qualcomm Life's biometric patches. Clinical validation is currently underway, and the patches are slated to be commercially available through Benchmark in 2018.
"We are thrilled to work with Qualcomm Life, a global leader in inventing revolutionary connected technologies, to create a new packaged sensor offering for health care companies that is economical, secure and user friendly," said Paul Tufano, CEO, Benchmark Electronics. "This collaboration aligns with our mission to deliver high-value solutions and innovative technology for our customers."
The innovative, cost-effective patches were developed on Qualcomm Life's 2net Design platform, a reference design platform for electronics modules which power connected medical devices, including disposable drug delivery devices and disposable diagnostic devices. 2net Design leverages Qualcomm's expertise in developing innovative reference designs for wireless, power-efficient, small-integrated modules to create smarter, more affordable and intuitive medical devices.