Business Wire05.05.17
A new system for automated drain line clearance used in electronic urine output (UO) monitoring of burn patients in intensive care settings shows promise in improving the accuracy of tracking real-time urine output and providing more precise data to guide fluid management and treatment, according to recent research.
Urine output is the key physiological measure that guides fluid therapy for burn patients and other critically ill patients in the intensive care unit, but the measurement of urine output can be inaccurate. More than 5.7 million patients are admitted to U.S. intensive care units (ICUs) each year, according to the Society of Critical Care Medicine.
“Improving how we measure UO at the bedside is vital in avoiding fluid overload, otherwise known as ‘fluid creep,’ which commonly leads to pulmonary edema, abdominal compartment syndrome and even death among critically ill patients,” said Dr. Daniel Burnett, a study author.
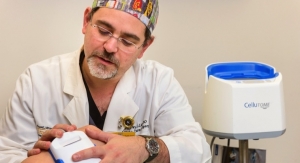
The study, funded in part through a National Institutes of Health grant, found that an early prototype of a new drain line clearance device called Accuryn Monitoring System1 provided multiple benefits, according to Burnett. Accuryn is being developed through a joint partnership with leading global healthcare manufacturer and distributor Medline Industries Inc., and medical device company Potrero Medical Inc.
“Our results showed that Accuryn, which features automatic real-time urine-drainage clearing every five minutes, eliminated trapped air that can cause urine retention in the bladder,” explained Burnett, who is Potrero’s CEO and an adjunct professor at the University of California at San Francisco. “In addition, this new drain line clearance system reduced the surges of urine flow that typically occur. Clinicians also recorded no instances of false oliguria, or decreased urine output, among test patients, both of which helped to improve the overall accuracy of urine tracking results.”
The new product also shows promise in enabling faster detection of kidney damage and can help lower hospital-acquired urinary-tract infections (CAUTI), and reduce the workload of caregivers, among other benefits, he added.
Involving patients from the University of Texas Medical Branch, Blocker Burn Unit, in Galveston, Texas, and the Joseph M. Still Burn Center at Doctors Hospital in Augusta, Ga., the study examined the extent of urine retention in the bladder from standard urine drain lines that is preventable, as well as the impact of Accuryn on urine output monitoring.
Standard UO measures are inaccurate because of imprecise time measurement methods and urine retention in the drain line system and bladder, explained Angela Zuick, R.N., BSN, CCRN, Medline clinical program manager and former ICU nurse manager.
“While standard burn unit clinical procedure requires that UO is measured every hour on the hour, this measurement is often not taken precisely at this time, due to the competing demands on nurses’ time in caring for seriously ill patients,” she said. “While some electronic monitors can be set to track urine output precisely on the hour, which eliminates timing issues, nurses often still need to ‘milk’ or manually manipulate the drainage tubing to keep urine flowing. This can skew the results and provide an inaccurate UO assessment that could negatively affect patient care, particularly if urine is retained in the bladder for hours at a time. In the study, Accuryn’s active drain line clearance system significantly reduced the impact of these issues.”
“Accuryn has enhanced patient care at our facility. It has changed how we measured urine output, allows for continuous monitoring and a more accurate measure on a moment-to-moment basis. It also shows high potential for detecting kidney damage,” said Dr. Bruce Friedman, a critical care medicine doctor who practices at the Joseph M. Still Burn Center at Doctors Hospital.
“Our initial promising results call for more research on the impact of this automated drain line clearing system in a wider population of patients where fluid levels and monitoring of urine output is critical,” noteed Burnett, an inventor with more than 200 patents and the developer of Accuryn.
Accuryn is a critical care monitoring system that transforms the traditional urinary catheter into the new standard of care in diagnostics. Accuryn uses proprietary electromechanical designs and advanced software algorithms to deliver automated, real-time actionable data including urine output, core body temperature and intra-abdominal pressure (IAP). The IAP measurement is taken directly from the catheter tip with just a push of a button, helping to reduce setup time, potential errors, and infection risk. Additionally, innovative active drain line clearance can overcome bladder retention issues that may make urine output measurements unreliable.
Medline is a global manufacturer and distributor serving the healthcare industry with medical supplies and clinical solutions that help customers achieve both clinical and financial success. Headquartered in Northfield, Ill., the company offers over 350,000 medical devices and support services through more than 1,200 direct sales representatives who are dedicated points of contact for customers across the continuum of care.
Potrero Medical Inc., the latest spinout of medical device incubator Theranova LLC, is headquartered in San Francisco, Calif. It was founded with a mission to improve outcomes, reduce costs and expand access to healthcare.
Reference:
1. The Accuryn System is a 510(k) cleared medical device available for sale in the United States.
Urine output is the key physiological measure that guides fluid therapy for burn patients and other critically ill patients in the intensive care unit, but the measurement of urine output can be inaccurate. More than 5.7 million patients are admitted to U.S. intensive care units (ICUs) each year, according to the Society of Critical Care Medicine.
“Improving how we measure UO at the bedside is vital in avoiding fluid overload, otherwise known as ‘fluid creep,’ which commonly leads to pulmonary edema, abdominal compartment syndrome and even death among critically ill patients,” said Dr. Daniel Burnett, a study author.
The study, funded in part through a National Institutes of Health grant, found that an early prototype of a new drain line clearance device called Accuryn Monitoring System1 provided multiple benefits, according to Burnett. Accuryn is being developed through a joint partnership with leading global healthcare manufacturer and distributor Medline Industries Inc., and medical device company Potrero Medical Inc.
“Our results showed that Accuryn, which features automatic real-time urine-drainage clearing every five minutes, eliminated trapped air that can cause urine retention in the bladder,” explained Burnett, who is Potrero’s CEO and an adjunct professor at the University of California at San Francisco. “In addition, this new drain line clearance system reduced the surges of urine flow that typically occur. Clinicians also recorded no instances of false oliguria, or decreased urine output, among test patients, both of which helped to improve the overall accuracy of urine tracking results.”
The new product also shows promise in enabling faster detection of kidney damage and can help lower hospital-acquired urinary-tract infections (CAUTI), and reduce the workload of caregivers, among other benefits, he added.
Involving patients from the University of Texas Medical Branch, Blocker Burn Unit, in Galveston, Texas, and the Joseph M. Still Burn Center at Doctors Hospital in Augusta, Ga., the study examined the extent of urine retention in the bladder from standard urine drain lines that is preventable, as well as the impact of Accuryn on urine output monitoring.
Standard UO measures are inaccurate because of imprecise time measurement methods and urine retention in the drain line system and bladder, explained Angela Zuick, R.N., BSN, CCRN, Medline clinical program manager and former ICU nurse manager.
“While standard burn unit clinical procedure requires that UO is measured every hour on the hour, this measurement is often not taken precisely at this time, due to the competing demands on nurses’ time in caring for seriously ill patients,” she said. “While some electronic monitors can be set to track urine output precisely on the hour, which eliminates timing issues, nurses often still need to ‘milk’ or manually manipulate the drainage tubing to keep urine flowing. This can skew the results and provide an inaccurate UO assessment that could negatively affect patient care, particularly if urine is retained in the bladder for hours at a time. In the study, Accuryn’s active drain line clearance system significantly reduced the impact of these issues.”
“Accuryn has enhanced patient care at our facility. It has changed how we measured urine output, allows for continuous monitoring and a more accurate measure on a moment-to-moment basis. It also shows high potential for detecting kidney damage,” said Dr. Bruce Friedman, a critical care medicine doctor who practices at the Joseph M. Still Burn Center at Doctors Hospital.
“Our initial promising results call for more research on the impact of this automated drain line clearing system in a wider population of patients where fluid levels and monitoring of urine output is critical,” noteed Burnett, an inventor with more than 200 patents and the developer of Accuryn.
Accuryn is a critical care monitoring system that transforms the traditional urinary catheter into the new standard of care in diagnostics. Accuryn uses proprietary electromechanical designs and advanced software algorithms to deliver automated, real-time actionable data including urine output, core body temperature and intra-abdominal pressure (IAP). The IAP measurement is taken directly from the catheter tip with just a push of a button, helping to reduce setup time, potential errors, and infection risk. Additionally, innovative active drain line clearance can overcome bladder retention issues that may make urine output measurements unreliable.
Medline is a global manufacturer and distributor serving the healthcare industry with medical supplies and clinical solutions that help customers achieve both clinical and financial success. Headquartered in Northfield, Ill., the company offers over 350,000 medical devices and support services through more than 1,200 direct sales representatives who are dedicated points of contact for customers across the continuum of care.
Potrero Medical Inc., the latest spinout of medical device incubator Theranova LLC, is headquartered in San Francisco, Calif. It was founded with a mission to improve outcomes, reduce costs and expand access to healthcare.
Reference:
1. The Accuryn System is a 510(k) cleared medical device available for sale in the United States.