Business Wire04.04.17
TransEnterix Inc., a company pioneering the use of robotics to improve minimally invasive surgery, has expanded the clinical adoption of the Senhance Robotic Surgical System to include a full range of hernia repair surgeries.
Hernia repairs represent one of the largest procedural opportunities for the Senhance. Millennium Research Group has estimated that during 2017, there will be a total of 1,080,400 hernia repair surgeries in Europe1 and 1,156,300 hernia repair surgeries in the United States.2
“Robotic hernia repair with the Senhance system represents a significant procedural area for our technology,” said Todd M. Pope, president and CEO of TransEnterix. “Hernia repairs, both inguinal and ventral, are amongst the most common surgical procedures performed worldwide. These procedures also represent one of the fastest growing uses of robotics in surgery. The introduction of Senhance to provide robotic assistance in these procedures brings a system with haptic feedback and attractive procedural costs to these operations for the first time.”
St. Marien-Krankenhaus Siegen in Germany, a major hernia repair center in Germany that performs more than 500 hernia repairs annually, was the first site to begin using the Senhance for unilateral and bilateral inguinal hernia repairs as well as ventral hernia repairs. Dr. Dietmar Stephan and Prof. Dr. Frank Willeke performed up to three robotic hernia surgeries per day with the Senhance during the first weeks of its clinical use at the hospital. This allows the team to adopt Senhance to include the day’s full operating schedule.
“We are pleased to offer robotic hernia repair utilizing the Senhance Robotic System, and all our operations utilizing this advanced technology have been performed with precision, safety and efficiency. The 3D visualization and precise control of the robotic instruments and camera are very helpful during delicate surgical tasks. The haptic feedback of the system is vital, and it allows me to feel the location of critical structures such as the pubic bone which aren’t always visible,” said Stephan, director of the Center for Minimally Invasive Surgery at St. Marien. “The Senhance is a significant progression in the field of minimally invasive hernia repair, and allows me to fully incorporate robotics into my hernia practice without having to justify high additional procedural costs.”
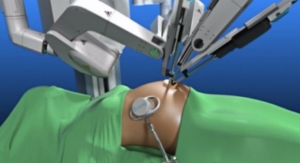
TransEnterix is focused on the commercialization of the Senhance Surgical Robotic System, a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology such as haptic feedback and eye sensing camera control. The company also developed the SurgiBot System, a single-port, robotically enhanced laparoscopic surgical platform. The Senhance Surgical Robotic System has been granted a CE Mark but is not currently available for sale in the United States.
References:
1. Millennium Research Group, Laparoscopic Devices Europe 2014 Market Analysis
2. Millennium Research Group, Medtech 360: Laparoscopic Devices US 2016 Market Analysis
Hernia repairs represent one of the largest procedural opportunities for the Senhance. Millennium Research Group has estimated that during 2017, there will be a total of 1,080,400 hernia repair surgeries in Europe1 and 1,156,300 hernia repair surgeries in the United States.2
“Robotic hernia repair with the Senhance system represents a significant procedural area for our technology,” said Todd M. Pope, president and CEO of TransEnterix. “Hernia repairs, both inguinal and ventral, are amongst the most common surgical procedures performed worldwide. These procedures also represent one of the fastest growing uses of robotics in surgery. The introduction of Senhance to provide robotic assistance in these procedures brings a system with haptic feedback and attractive procedural costs to these operations for the first time.”
St. Marien-Krankenhaus Siegen in Germany, a major hernia repair center in Germany that performs more than 500 hernia repairs annually, was the first site to begin using the Senhance for unilateral and bilateral inguinal hernia repairs as well as ventral hernia repairs. Dr. Dietmar Stephan and Prof. Dr. Frank Willeke performed up to three robotic hernia surgeries per day with the Senhance during the first weeks of its clinical use at the hospital. This allows the team to adopt Senhance to include the day’s full operating schedule.
“We are pleased to offer robotic hernia repair utilizing the Senhance Robotic System, and all our operations utilizing this advanced technology have been performed with precision, safety and efficiency. The 3D visualization and precise control of the robotic instruments and camera are very helpful during delicate surgical tasks. The haptic feedback of the system is vital, and it allows me to feel the location of critical structures such as the pubic bone which aren’t always visible,” said Stephan, director of the Center for Minimally Invasive Surgery at St. Marien. “The Senhance is a significant progression in the field of minimally invasive hernia repair, and allows me to fully incorporate robotics into my hernia practice without having to justify high additional procedural costs.”
TransEnterix is focused on the commercialization of the Senhance Surgical Robotic System, a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology such as haptic feedback and eye sensing camera control. The company also developed the SurgiBot System, a single-port, robotically enhanced laparoscopic surgical platform. The Senhance Surgical Robotic System has been granted a CE Mark but is not currently available for sale in the United States.
References:
1. Millennium Research Group, Laparoscopic Devices Europe 2014 Market Analysis
2. Millennium Research Group, Medtech 360: Laparoscopic Devices US 2016 Market Analysis