Clinical Innovations04.03.17
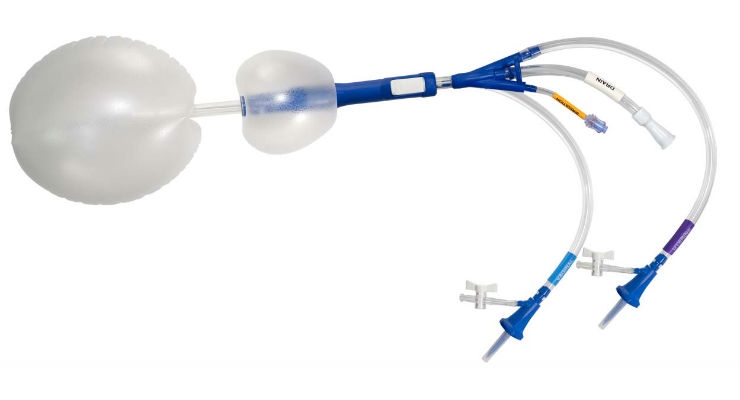
Clinical Innovations, one of the largest medical device companies exclusively focused on labor and delivery and the neonatal intensive care unit, has announced the official launch of the ebb Complete Tamponade System, which is an effective treatment for postpartum hemorrhage (PPH).
Postpartum hemorrhage is one of the leading causes of maternal mortality. As part of Clinical Innovations’ mission to improve the lives of mothers and their babies throughout the world, ebb allows for rapid, emergency deployment to significantly reduce or eliminate bleeding in up to 98 percent of patients.1
With its unique, malleable polyurethane material and patented dual-balloon design, ebb conforms to the patient’s anatomy and can help avoid hysterectomy, according to a large prospective study published in the American Journal of Obstetrics and Gynecology.
“Postpartum hemorrhage puts up to 200,000 mothers in the U.S. at risk every year and can be fatal in hours if untreated. The ebb Complete Tamponade System’s rapid-response design is an easy-to-deploy, cost-effective solution for improving patient outcomes associated with PPH,” said Dr. Ross McQuivey, chief medical officer for Clinical Innovations. “We’re committed to developing safe, reliable and innovative solutions to improve the lives of mothers and their babies, and we’re proud to have ebb return as a core part of our growing labor and delivery product portfolio.”
After receiving FDA clearance in January of 2016, Clinical Innovations conducted a successful limited release where ebb has been used in hospitals throughout the United States and Europe for the past year. “We have had great feedback on ebb and excellent clinical results for the treatment of PPH. We are excited to launch ebb so clinicians can utilize this critical device to save lives throughout the world,” said Jeff Bradford, vice president global marketing.
1 Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Dildy GA, Belfort MA, Adair D, et al. Am J Obstet Gynecol. 2014;210:136.e1-6.
Postpartum hemorrhage is one of the leading causes of maternal mortality. As part of Clinical Innovations’ mission to improve the lives of mothers and their babies throughout the world, ebb allows for rapid, emergency deployment to significantly reduce or eliminate bleeding in up to 98 percent of patients.1
With its unique, malleable polyurethane material and patented dual-balloon design, ebb conforms to the patient’s anatomy and can help avoid hysterectomy, according to a large prospective study published in the American Journal of Obstetrics and Gynecology.
“Postpartum hemorrhage puts up to 200,000 mothers in the U.S. at risk every year and can be fatal in hours if untreated. The ebb Complete Tamponade System’s rapid-response design is an easy-to-deploy, cost-effective solution for improving patient outcomes associated with PPH,” said Dr. Ross McQuivey, chief medical officer for Clinical Innovations. “We’re committed to developing safe, reliable and innovative solutions to improve the lives of mothers and their babies, and we’re proud to have ebb return as a core part of our growing labor and delivery product portfolio.”
After receiving FDA clearance in January of 2016, Clinical Innovations conducted a successful limited release where ebb has been used in hospitals throughout the United States and Europe for the past year. “We have had great feedback on ebb and excellent clinical results for the treatment of PPH. We are excited to launch ebb so clinicians can utilize this critical device to save lives throughout the world,” said Jeff Bradford, vice president global marketing.
1 Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Dildy GA, Belfort MA, Adair D, et al. Am J Obstet Gynecol. 2014;210:136.e1-6.