Medtronic02.24.17
Data presented on Medtronic's Solitaire stent retriever at the International Stroke Conference (ISC) in Houston demonstrate that the results from the four pivotal randomized controlled trials—SOLITAIRE FR With the Intention For Thrombectomy as PRIMary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME), Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE), EXtending the Time for Thrombolysis in Emergency Neurological Deficits—Intra-Arterial (EXTEND IA), and Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours (REVASCAT)—can be replicated in U.S. centers, in a pragmatic, real-world setting reconfirming the benefits of mechanical thrombectomy in patients suffering acute ischemic stroke (AIS). Study investigators presented the results from Systematic Evaluation of Patients TReated with Neurothrombectomy Devices for AcuTe Ischemic Stroke (STRATIS) Registry.
STRATIS is the largest AIS study to date with a focus on the impact of systems of care on clinical outcomes. STRATIS examined the impact of treatment delays on patient outcomes when treated with the Solitaire stent retriever and intravenous tissue plasminogen activator (IV-tPA), if eligible. In the study, 984 patients were enrolled at 55 centers throughout the U.S.; 64 percent were treated with the Solitaire stent retriever and IV-tPA and 36 percent were treated with the Solitaire stent retriever alone. The study found that interhospital transfer was associated with significant delays to treatment and significantly lower chance of functional independence at 90 days (60.0 percent vs. 52.2 percent, p=0.02). Further, those treated via balloon guide catheter (BGC) had higher rates of functional independence at 90 days (61.8 percent vs. 50.2 percent, p=0.002) with fewer passes (1.7 vs. 2.0, p=0.0008) than patients treated via distal access catheter.
"The STRATIS registry confirms that the outcomes from four of the global randomized clinical trials that helped to transform stroke treatment are applicable in different health systems across the U.S. with the same positive results," said Curtis Given, M.D., co-director, Neurointerventional Services, Baptist Health, Lexington, Ky. "We are consistently seeing that access to stent retrievers reduces long-term disability in patients. We must continue to work towards a system that makes early treatment with this technology available to all patients."
In addition to confirming real-world application, a recent study published in Stroke, Cost-Effectiveness of Solitaire Stent Retriever Thrombectomy for Acute Ischemic Stroke: Results from the SWIFT-PRIME Trial, found that treatment with both the Solitaire stent retriever and IV-tPA is highly cost-effective and an economically dominant strategy with substantial long-term cost savings and gains in both life-expectancy and quality-adjusted life-expectancy compared to IV-tPA alone. While initial costs were higher for the Solitaire stent retriever and IV-tPA compared to IV-tPA alone ($45,761 vs. $28,578, p<0.001), costs between patient discharge and 90 days, were $4,904 per patient lower ($11,270 vs. $16,174, p=0.014) for patients treated with the Solitaire stent retriever than patients treated with IV-tPA alone due to significant reductions in rehospitalization, rehabilitation-related, and long-term nursing home costs. Finally, treatment with the Solitaire stent retriever was associated with cost savings of $23,203 per patient over a lifetime.
"The cost-effectiveness data shows that despite higher initial treatment costs, in the long run, patients who receive stent retriever therapy with the Solitaire stent retriever spend less time in the hospital, less time in rehabilitation, and less time in nursing home care after an acute ischemic stroke. Patients have faster and complete recoveries, and the healthcare system saves money overall," said Jeffrey L. Saver, M.D., FAHA, FAAN, FANA, professor of neurology, Geffen School of Medicine at the University of California, Los Angeles and director, UCLA Comprehensive Stroke Center.
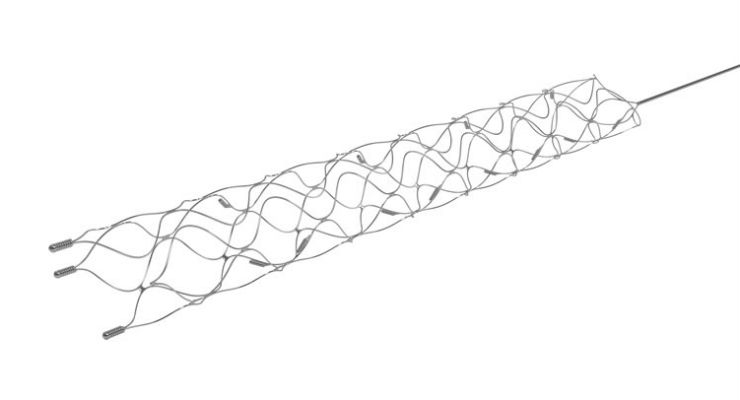
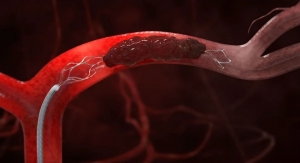
The Solitaire stent retriever uses a micro-sized catheter to access arteries in the brain, helping to restore blood flow and remove large blood clots causing AIS.
"Medtronic continues to provide ground-breaking data that shows the impact and now, long-term cost savings of our Solitaire stent retriever, the most studied of this class of devices," said Stacey Pugh, vice president and general manager of Medtronic's Neurovascular business, which is part of the Restorative Therapies Group. "As the pioneer of stent retriever technology, we are committed to working with hospitals, providers and organizations such as the American Heart Association/American Stroke Association (AHA/ASA) to continue to bring the most effective stroke treatments to the patients who need it most."
According to the American Heart Association/American Stroke Association (AHA/ASA), stroke is the fifth leading cause of death in the U.S. and a leading cause of disability. In June 2015, the AHA/ASA published new stroke treatment guidelines that recommended the use of stent retriever technology—such as the Solitaire stent retriever device—in conjunction with IV rtPA/alteplase as a first-line treatment for eligible patients.
STRATIS is the largest AIS study to date with a focus on the impact of systems of care on clinical outcomes. STRATIS examined the impact of treatment delays on patient outcomes when treated with the Solitaire stent retriever and intravenous tissue plasminogen activator (IV-tPA), if eligible. In the study, 984 patients were enrolled at 55 centers throughout the U.S.; 64 percent were treated with the Solitaire stent retriever and IV-tPA and 36 percent were treated with the Solitaire stent retriever alone. The study found that interhospital transfer was associated with significant delays to treatment and significantly lower chance of functional independence at 90 days (60.0 percent vs. 52.2 percent, p=0.02). Further, those treated via balloon guide catheter (BGC) had higher rates of functional independence at 90 days (61.8 percent vs. 50.2 percent, p=0.002) with fewer passes (1.7 vs. 2.0, p=0.0008) than patients treated via distal access catheter.
"The STRATIS registry confirms that the outcomes from four of the global randomized clinical trials that helped to transform stroke treatment are applicable in different health systems across the U.S. with the same positive results," said Curtis Given, M.D., co-director, Neurointerventional Services, Baptist Health, Lexington, Ky. "We are consistently seeing that access to stent retrievers reduces long-term disability in patients. We must continue to work towards a system that makes early treatment with this technology available to all patients."
In addition to confirming real-world application, a recent study published in Stroke, Cost-Effectiveness of Solitaire Stent Retriever Thrombectomy for Acute Ischemic Stroke: Results from the SWIFT-PRIME Trial, found that treatment with both the Solitaire stent retriever and IV-tPA is highly cost-effective and an economically dominant strategy with substantial long-term cost savings and gains in both life-expectancy and quality-adjusted life-expectancy compared to IV-tPA alone. While initial costs were higher for the Solitaire stent retriever and IV-tPA compared to IV-tPA alone ($45,761 vs. $28,578, p<0.001), costs between patient discharge and 90 days, were $4,904 per patient lower ($11,270 vs. $16,174, p=0.014) for patients treated with the Solitaire stent retriever than patients treated with IV-tPA alone due to significant reductions in rehospitalization, rehabilitation-related, and long-term nursing home costs. Finally, treatment with the Solitaire stent retriever was associated with cost savings of $23,203 per patient over a lifetime.
"The cost-effectiveness data shows that despite higher initial treatment costs, in the long run, patients who receive stent retriever therapy with the Solitaire stent retriever spend less time in the hospital, less time in rehabilitation, and less time in nursing home care after an acute ischemic stroke. Patients have faster and complete recoveries, and the healthcare system saves money overall," said Jeffrey L. Saver, M.D., FAHA, FAAN, FANA, professor of neurology, Geffen School of Medicine at the University of California, Los Angeles and director, UCLA Comprehensive Stroke Center.
The Solitaire stent retriever uses a micro-sized catheter to access arteries in the brain, helping to restore blood flow and remove large blood clots causing AIS.
"Medtronic continues to provide ground-breaking data that shows the impact and now, long-term cost savings of our Solitaire stent retriever, the most studied of this class of devices," said Stacey Pugh, vice president and general manager of Medtronic's Neurovascular business, which is part of the Restorative Therapies Group. "As the pioneer of stent retriever technology, we are committed to working with hospitals, providers and organizations such as the American Heart Association/American Stroke Association (AHA/ASA) to continue to bring the most effective stroke treatments to the patients who need it most."
According to the American Heart Association/American Stroke Association (AHA/ASA), stroke is the fifth leading cause of death in the U.S. and a leading cause of disability. In June 2015, the AHA/ASA published new stroke treatment guidelines that recommended the use of stent retriever technology—such as the Solitaire stent retriever device—in conjunction with IV rtPA/alteplase as a first-line treatment for eligible patients.