MMJ Labs02.24.17
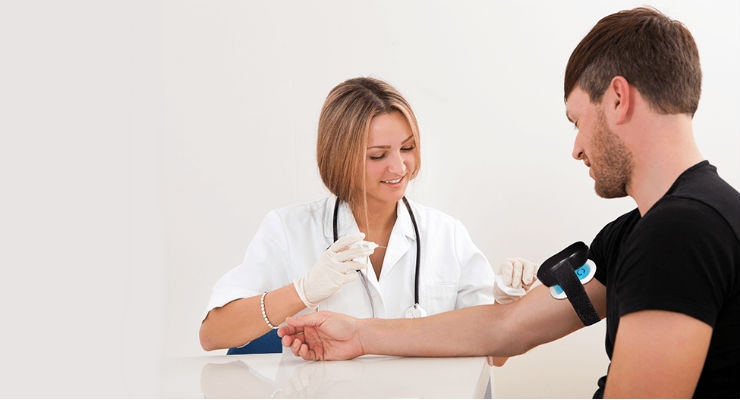
New research from Children’s Hospital of Philadelphia found a novel neuromodulation pain relief device “Buzzy” made IV access in the Emergency Department (ED) 10 times faster than standard IV access pain relief cream. Buzzy is an FDA 510K cleared cold and vibration device indicated for controlling needle pain.
Initially intended for children, the striped bee design isn’t what is typically expected for a regulated medical device. While both IV access groups had equivalent patient pain relief, as well as nursing and parent satisfaction, IVs placed with Buzzy were significantly faster.
“The time for lab draws and IV access can be the biggest barrier to throughput and speed in the ED,” said Dr. Amy Baxter, emergency pediatrician and inventor of Buzzy. “To be able to get labs in one trip rather than going back and forth to a room to place a cream is much more efficient.”
Buzzy was previously proven twice as effective at pain relief as another leading anesthetic, cold spray, with three times the first-attempt success. Since a 2014 review recommended against using cold spray, hospitals wanting a fast acting pain reliever have looked for other options.
“CHOP is truly an innovator,” noted Baxter. “They were willing to think outside the box and formally investigate a vibrating bee versus a pharmaceutical product for pain. We’re thrilled with the results. Not only is Buzzy 10 times faster, but it provides a significant savings to the department.” Disposable ice packs and Buzzy cost approximately $2.77 per participant compared to a $4-6 typical cost of topical anesthetics.
Patient experience has been a priority for medical systems over the past decade, but is particularly important for emergency procedures in children. Young children now get 30+ injections with routine vaccinations, so more patients come to emergency departments fearing needles. A fearful older child can present a risk to staff, so relieving pain and anxiety is a priority.
The study randomized 251 patients to Buzzy or topical anesthetic, and will appear in the journal Pediatric Emergency Care. Buzzy is made by MMJ Labs Atlanta GA, industry innovators in noninvasive pain relief.
Initially intended for children, the striped bee design isn’t what is typically expected for a regulated medical device. While both IV access groups had equivalent patient pain relief, as well as nursing and parent satisfaction, IVs placed with Buzzy were significantly faster.
“The time for lab draws and IV access can be the biggest barrier to throughput and speed in the ED,” said Dr. Amy Baxter, emergency pediatrician and inventor of Buzzy. “To be able to get labs in one trip rather than going back and forth to a room to place a cream is much more efficient.”
Buzzy was previously proven twice as effective at pain relief as another leading anesthetic, cold spray, with three times the first-attempt success. Since a 2014 review recommended against using cold spray, hospitals wanting a fast acting pain reliever have looked for other options.
“CHOP is truly an innovator,” noted Baxter. “They were willing to think outside the box and formally investigate a vibrating bee versus a pharmaceutical product for pain. We’re thrilled with the results. Not only is Buzzy 10 times faster, but it provides a significant savings to the department.” Disposable ice packs and Buzzy cost approximately $2.77 per participant compared to a $4-6 typical cost of topical anesthetics.
Patient experience has been a priority for medical systems over the past decade, but is particularly important for emergency procedures in children. Young children now get 30+ injections with routine vaccinations, so more patients come to emergency departments fearing needles. A fearful older child can present a risk to staff, so relieving pain and anxiety is a priority.
The study randomized 251 patients to Buzzy or topical anesthetic, and will appear in the journal Pediatric Emergency Care. Buzzy is made by MMJ Labs Atlanta GA, industry innovators in noninvasive pain relief.