Business Wire12.20.16
W. L. Gore & Associates announced that GORE SYNECOR Biomaterial for hernia repair has received the Innovative Technology designation from Vizient Inc., the largest member-driven healthcare performance improvement company in America. The designation was based on reviews of GORE SYNECOR Biomaterial by hospital experts who attended the Vizient Innovative Technology Exchange in September 2016.
The event provided medical technology suppliers the opportunity to demonstrate their product and gain direct feedback from 1,300 onsite clinical experts and healthcare providers on the impact their products may have on improving clinical care, safety, or benefits to an organization’s care and business model.
For years surgeons in hernia repair faced a choice between a permanent synthetic mesh, offering strength but potentially higher complication risks, or a bioabsorbable mesh (biosynthetic or biologic) with lower complication risks, but also leading to possibly higher recurrence rates and the need for multi-stage procedures.
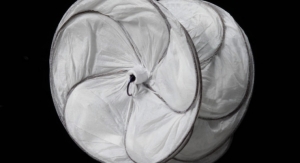
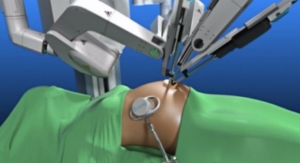
Constructed with GORE BIO-A web scaffold combined with a permanent knit of dense monofilament PTFE fiber that may reduce the risk of harboring bacteria, GORE SYNECOR Biomaterial provides both high strength and rapid vascularity, for durable, lasting repairs. The non-porous, bioabsorbable film minimizes tissue attachment at the visceral side, making it an ideal product for intraperitoneal positioning, laparoscopic and robotic use.
“GORE SYNECOR Biomaterial was engineered to be a unique solution in delivering a single-stage hernia repair,” explained David Lane, business unit leader for general medical products at W. L. Gore & Associates. “Our goal is to work with hospitals and surgeons in meeting their unsolved needs while delivering clear value and promoting optimal outcomes for patients.”
“Due to the number of products and services being released and marketed as ‘innovative’, member hospitals truly value the peer-review system in place at Vizient to help them identify products worth further evaluation at their own facilities,” said Debbie Archer, director of procurement and leader of Vizient’s Innovative Technology program for suppliers. “After a review of the GORE SYNECOR Biomaterial, Vizient’s members agreed this product offers unique and incremental benefit over other products available on the market today, and recommended it for an Innovative Technology designation.”
Vizient Inc. represents the combined strengths of the organizations formerly known as VHA Inc., University HealthSystem Consortium, Novation and MedAssets’ Spend and Clinical Resource Management. Since 2003, nearly 2,200 new products and technologies have been submitted through the Vizient Innovative Technology program. Vizient works with member-led councils and task forces to identify and review potentially innovative products. If it is determined that a product is innovative, a contract may be awarded outside of Vizient’s competitive bid cycle.
Gore Medical's family of products includes vascular grafts, endovascular and interventional devices, surgical meshes for hernia and soft tissue reconstruction, staple line reinforcement materials, and sutures for use in vascular, cardiac, and general surgery. The company is headquartered in Flagstaff, Ariz.
The event provided medical technology suppliers the opportunity to demonstrate their product and gain direct feedback from 1,300 onsite clinical experts and healthcare providers on the impact their products may have on improving clinical care, safety, or benefits to an organization’s care and business model.
For years surgeons in hernia repair faced a choice between a permanent synthetic mesh, offering strength but potentially higher complication risks, or a bioabsorbable mesh (biosynthetic or biologic) with lower complication risks, but also leading to possibly higher recurrence rates and the need for multi-stage procedures.
Constructed with GORE BIO-A web scaffold combined with a permanent knit of dense monofilament PTFE fiber that may reduce the risk of harboring bacteria, GORE SYNECOR Biomaterial provides both high strength and rapid vascularity, for durable, lasting repairs. The non-porous, bioabsorbable film minimizes tissue attachment at the visceral side, making it an ideal product for intraperitoneal positioning, laparoscopic and robotic use.
“GORE SYNECOR Biomaterial was engineered to be a unique solution in delivering a single-stage hernia repair,” explained David Lane, business unit leader for general medical products at W. L. Gore & Associates. “Our goal is to work with hospitals and surgeons in meeting their unsolved needs while delivering clear value and promoting optimal outcomes for patients.”
“Due to the number of products and services being released and marketed as ‘innovative’, member hospitals truly value the peer-review system in place at Vizient to help them identify products worth further evaluation at their own facilities,” said Debbie Archer, director of procurement and leader of Vizient’s Innovative Technology program for suppliers. “After a review of the GORE SYNECOR Biomaterial, Vizient’s members agreed this product offers unique and incremental benefit over other products available on the market today, and recommended it for an Innovative Technology designation.”
Vizient Inc. represents the combined strengths of the organizations formerly known as VHA Inc., University HealthSystem Consortium, Novation and MedAssets’ Spend and Clinical Resource Management. Since 2003, nearly 2,200 new products and technologies have been submitted through the Vizient Innovative Technology program. Vizient works with member-led councils and task forces to identify and review potentially innovative products. If it is determined that a product is innovative, a contract may be awarded outside of Vizient’s competitive bid cycle.
Gore Medical's family of products includes vascular grafts, endovascular and interventional devices, surgical meshes for hernia and soft tissue reconstruction, staple line reinforcement materials, and sutures for use in vascular, cardiac, and general surgery. The company is headquartered in Flagstaff, Ariz.