PR Newswire11.02.16
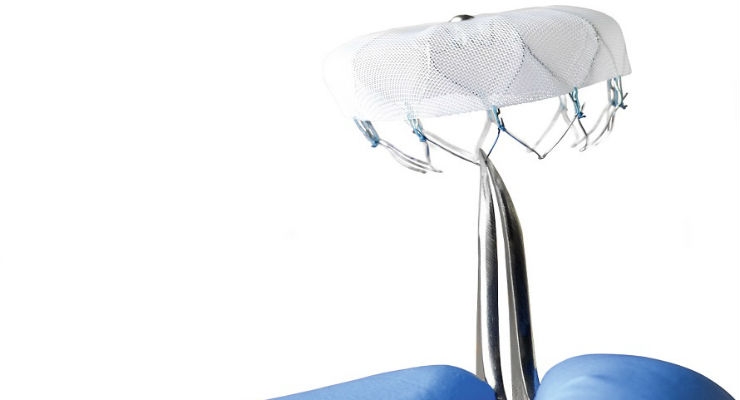
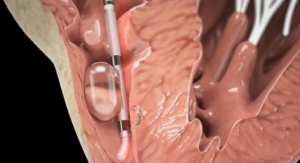
Boston Scientific announced initial U.S. commercial performance results of the WATCHMAN Left Atrial Appendage Closure (LAAC) Device during a late-breaking clinical trial session at the 28th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Washington, D.C. Data demonstrated a high rate of procedural success and low rate of complications for the device which offers stroke risk reduction for patients with non-valvular atrial fibrillation (AF) who are seeking an alternative to long-term warfarin therapy. The study was also published online today in the Journal of the American College of Cardiology.
In advance of a formal national clinical registry becoming available, procedural data were collected via WATCHMAN clinical specialists during 3,822 consecutive LAAC procedures performed between March 2015 and May 2016 by 382 operating physicians at 169 U.S. medical centers. In this case series, there was a 95.6% implant success rate with a median procedure time of 50 minutes.
Half of the procedures were performed by new implanting physicians without previous experience with the device. Nevertheless, the overall rate of complications evaluated within these data was low at 1.63%, and compared favorably to the clinical trial data leading to device approval, validating the rigorous process for selecting and training new operators. Pericardial tamponade requiring intervention was the most frequent major procedural complication, which was seen in 1.02% of patients. In an additional 0.29% of patients, a hemodynamically insignificant pericardial effusion was observed that did not require intervention. Device embolization, procedure-related stroke and mortality rates also remained low at 0.24%, 0.08% and 0.08%, respectively.
"The 'real-world' data collected from this study indicate high procedural success, even with the large number of new implanting physicians performing one-half of the procedures," said Vivek Reddy, M.D., co-principal investigator and director of Cardiac Arrhythmia Services for The Mount Sinai Hospital and the Mount Sinai Health System. "More importantly, we confirmed the safety of this therapy as evidenced by the low rate of complications."
"This evaluation includes the largest WATCHMAN device patient population studied to date and demonstrated low and consistent complication rates compared to those seen in previous clinical trials," said David R. Holmes, M.D., co-principal investigator and professor of medicine at Mayo Clinic College of Medicine and a consultant in the Division of Cardiovascular Diseases and the Department of Internal Medicine at Mayo Clinic in Rochester, Minn. "This provides important procedural insights in the absence of an official Centers for Medicare and Medicaid Services registry being available to collect data immediately following device approval by the U.S. Food and Drug Administration in March, 2015."
The formal national registry capturing data on left atrial appendage occlusion (LAAO) procedures—the LAAO Registry, sponsored by the American College of Cardiology – was approved in August, 2016 by the Centers for Medicare and Medicaid Services. Participation in the registry is a condition of coverage as outlined in the LAAC National Coverage Determination (NCD). The NCD was effective February 8, 2016 and fully implemented nationwide on October 3, 2016.
In advance of a formal national clinical registry becoming available, procedural data were collected via WATCHMAN clinical specialists during 3,822 consecutive LAAC procedures performed between March 2015 and May 2016 by 382 operating physicians at 169 U.S. medical centers. In this case series, there was a 95.6% implant success rate with a median procedure time of 50 minutes.
Half of the procedures were performed by new implanting physicians without previous experience with the device. Nevertheless, the overall rate of complications evaluated within these data was low at 1.63%, and compared favorably to the clinical trial data leading to device approval, validating the rigorous process for selecting and training new operators. Pericardial tamponade requiring intervention was the most frequent major procedural complication, which was seen in 1.02% of patients. In an additional 0.29% of patients, a hemodynamically insignificant pericardial effusion was observed that did not require intervention. Device embolization, procedure-related stroke and mortality rates also remained low at 0.24%, 0.08% and 0.08%, respectively.
"The 'real-world' data collected from this study indicate high procedural success, even with the large number of new implanting physicians performing one-half of the procedures," said Vivek Reddy, M.D., co-principal investigator and director of Cardiac Arrhythmia Services for The Mount Sinai Hospital and the Mount Sinai Health System. "More importantly, we confirmed the safety of this therapy as evidenced by the low rate of complications."
"This evaluation includes the largest WATCHMAN device patient population studied to date and demonstrated low and consistent complication rates compared to those seen in previous clinical trials," said David R. Holmes, M.D., co-principal investigator and professor of medicine at Mayo Clinic College of Medicine and a consultant in the Division of Cardiovascular Diseases and the Department of Internal Medicine at Mayo Clinic in Rochester, Minn. "This provides important procedural insights in the absence of an official Centers for Medicare and Medicaid Services registry being available to collect data immediately following device approval by the U.S. Food and Drug Administration in March, 2015."
The formal national registry capturing data on left atrial appendage occlusion (LAAO) procedures—the LAAO Registry, sponsored by the American College of Cardiology – was approved in August, 2016 by the Centers for Medicare and Medicaid Services. Participation in the registry is a condition of coverage as outlined in the LAAC National Coverage Determination (NCD). The NCD was effective February 8, 2016 and fully implemented nationwide on October 3, 2016.