Business Wire09.26.16
BrainScope Company Inc. announced today that the United States Food and Drug Administration (FDA) has cleared the company to market the Ahead 300, its most advanced medical device for use in assessing traumatic brain injury (TBI). Developed in partnership with the U.S. Department of Defense, the Ahead 300 provides a multi-modal device of clinically relevant measures, offering clinicians a comprehensive panel of data to assist in their diagnosis of the full spectrum of TBI, including concussion. The Ahead 300 represents an evolution from the three BrainScope products that have previously received FDA clearance, and with its substantial additional capabilities, will be the first product the company will sell commercially.
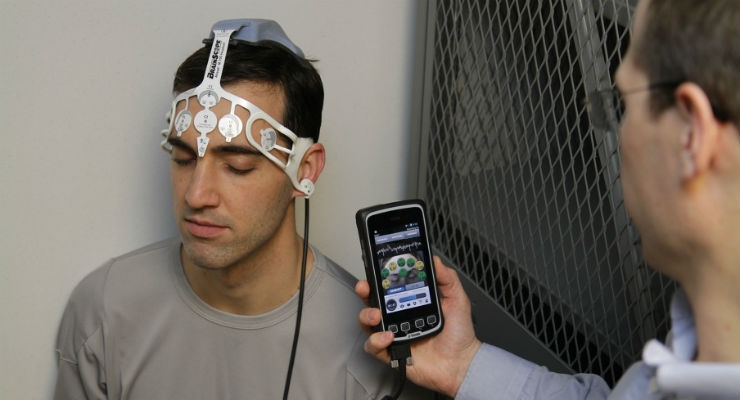
The Ahead 300 features BrainScope’s proprietary, patent-protected electroencephalography (EEG) capabilities utilizing sophisticated algorithms and machine learning to analyze head-injured patient data. Leveraging state-of-the-art handheld smartphone technology and a proprietary disposable electrode headset, the Ahead 300 provides a rapid, objective assessment of the likelihood of the presence of TBI in patients who present with mild symptoms at the point of care. In addition to EEG capabilities, the Ahead 300 includes additional assessments providing clinicians with a digitized, streamlined report, delivering a comprehensive and objective panel of results to facilitate their differential diagnosis.
“FDA clearance of the Ahead 300 is a bellwether moment in our company’s history. The Ahead 300 provides the specific capabilities needed today for the clinician to undertake a comprehensive assessment addressing the full spectrum of traumatic brain injury, from structural injuries visible on a CT scan, through mild TBI, also known as concussion,” stated Michael Singer, Chief Executive Officer of BrainScope.
According to recent publications, 95% of all head-injured patients who go to the emergency department present with mild symptoms. The Ahead 300 will help health care professionals clinically diagnose the entire spectrum of TBI in vitally important healthcare settings, including hospital emergency departments, urgent care centers and concussion clinics, and throughout the military healthcare system. With over five million U.S. patients seen each year with closed head injuries, and millions more who do not seek evaluation, the Ahead 300 allows for head-injured patients to be objectively assessed at the point of care, including locations of intercollegiate and professional sports, thereby significantly improving triage, medical management, and the ultimate clinical diagnosis. The ability to rapidly identify TBI can meaningfully reduce the morbidity and mortality of head-injured patients.
“The BrainScope platform is a desperately needed addition to the medical provider's clinical armamentarium. With mild TBI as prevalent as it is, an objective easy-to-use point of care device to identify those patients at highest risk of concussion is a critical need,” added Geoffrey Ling, MD, COL, USA (Ret.).
“Our FDA clearance represents many years of development with our partner, the Department of Defense. We are particularly grateful for the strong, continuing partnership which has led to this outstanding outcome,” noted Singer. Since 2011, BrainScope has been awarded over $27 million of research contracts by the U.S. Department of Defense for research and development of the Ahead system. These contracts supported the evolution and enhancement of BrainScope’s Ahead technology and nationwide, multi-site clinical studies in hospital emergency departments.
BrainScope has focused on developing medical technology substantiated by clinical evidence emanating from over 20 clinical studies at 55 clinical sites over the past eight years. Stemming from this substantial clinical work, there is an extensive and growing bibliography of peer-reviewed publications underpinning the BrainScope technology. BrainScope has also developed a broad Intellectual Property portfolio now consisting of 100 issued and pending patents worldwide.
“We are now preparing to commercialize the Ahead 300, which we expect to be available in the coming months. We will be making a formal announcement upon our commercial launch of the Ahead 300,” commented Singer.
The Ahead 300 features BrainScope’s proprietary, patent-protected electroencephalography (EEG) capabilities utilizing sophisticated algorithms and machine learning to analyze head-injured patient data. Leveraging state-of-the-art handheld smartphone technology and a proprietary disposable electrode headset, the Ahead 300 provides a rapid, objective assessment of the likelihood of the presence of TBI in patients who present with mild symptoms at the point of care. In addition to EEG capabilities, the Ahead 300 includes additional assessments providing clinicians with a digitized, streamlined report, delivering a comprehensive and objective panel of results to facilitate their differential diagnosis.
“FDA clearance of the Ahead 300 is a bellwether moment in our company’s history. The Ahead 300 provides the specific capabilities needed today for the clinician to undertake a comprehensive assessment addressing the full spectrum of traumatic brain injury, from structural injuries visible on a CT scan, through mild TBI, also known as concussion,” stated Michael Singer, Chief Executive Officer of BrainScope.
According to recent publications, 95% of all head-injured patients who go to the emergency department present with mild symptoms. The Ahead 300 will help health care professionals clinically diagnose the entire spectrum of TBI in vitally important healthcare settings, including hospital emergency departments, urgent care centers and concussion clinics, and throughout the military healthcare system. With over five million U.S. patients seen each year with closed head injuries, and millions more who do not seek evaluation, the Ahead 300 allows for head-injured patients to be objectively assessed at the point of care, including locations of intercollegiate and professional sports, thereby significantly improving triage, medical management, and the ultimate clinical diagnosis. The ability to rapidly identify TBI can meaningfully reduce the morbidity and mortality of head-injured patients.
“The BrainScope platform is a desperately needed addition to the medical provider's clinical armamentarium. With mild TBI as prevalent as it is, an objective easy-to-use point of care device to identify those patients at highest risk of concussion is a critical need,” added Geoffrey Ling, MD, COL, USA (Ret.).
“Our FDA clearance represents many years of development with our partner, the Department of Defense. We are particularly grateful for the strong, continuing partnership which has led to this outstanding outcome,” noted Singer. Since 2011, BrainScope has been awarded over $27 million of research contracts by the U.S. Department of Defense for research and development of the Ahead system. These contracts supported the evolution and enhancement of BrainScope’s Ahead technology and nationwide, multi-site clinical studies in hospital emergency departments.
BrainScope has focused on developing medical technology substantiated by clinical evidence emanating from over 20 clinical studies at 55 clinical sites over the past eight years. Stemming from this substantial clinical work, there is an extensive and growing bibliography of peer-reviewed publications underpinning the BrainScope technology. BrainScope has also developed a broad Intellectual Property portfolio now consisting of 100 issued and pending patents worldwide.
“We are now preparing to commercialize the Ahead 300, which we expect to be available in the coming months. We will be making a formal announcement upon our commercial launch of the Ahead 300,” commented Singer.