U.S. Food and Drug Administration09.14.16
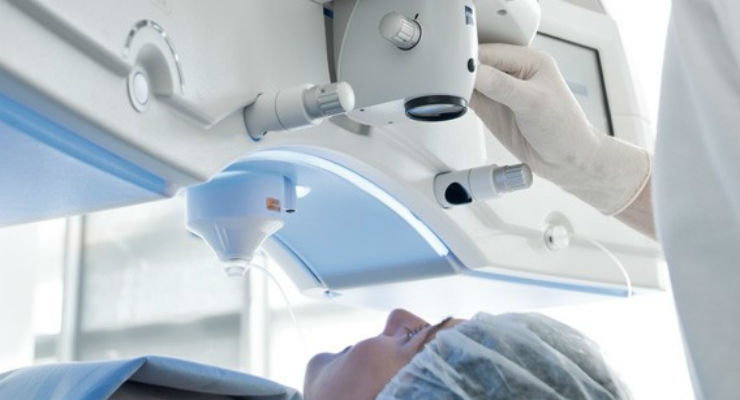
The U.S. Food and Drug Administration approved the VisuMax Femtosecond Laser for the small incision lenticule extraction (SMILE) procedure to reduce or eliminate nearsightedness in certain patients 22 years of age or older.
Not all patients are candidates for SMILE, and individuals should carefully review the patient labeling and discuss their expectations with their eye care professional.
“This approval expands the surgical treatment options available to patients for correcting nearsightedness,” said Malvina Eydelman, M.D., director of Ophthalmic and Ear, Nose and Throat Devices, in FDA’s Center for Devices and Radiological Health.
Nearsightedness, or myopia, is a common vision condition in which close objects are seen clearly, but objects farther away are blurred. It occurs when the eye focuses light in front of the retina. This can be due to the shape of the cornea being too steep and/or the length of the eyeball being too long.
The VisuMax Femtosecond Laser removes a small amount of eye tissue to permanently reshape the cornea. A femtosecond (very fast, short-pulsed) laser makes cuts within the cornea, creating a disc-shaped piece of tissue that is removed by the surgeon through a small incision in the surface of the cornea. This tissue removal causes the shape of the cornea to change, which corrects the nearsightedness.
A clinical study of the safety and effectiveness of the device to correct nearsightedness found the procedure resulted in stable vision correction at six months. Of the 328 participants evaluated at six months, all but one had uncorrected (without glasses or contacts) visual acuity of 20/40 or better, and 88 percent had uncorrected visual acuity of 20/20 or better.
Common complications during surgery included difficulty removing the corneal tissue and loss of suction that keeps the laser aligned with your eye. Common complications after surgery included debris at the site of tissue removal, dry eye, moderate to severe glare and moderate to severe halos.
The VisuMax Femtosecond Laser is manufactured by Carl Zeiss Meditec Inc., of Dublin, Calif.
Not all patients are candidates for SMILE, and individuals should carefully review the patient labeling and discuss their expectations with their eye care professional.
“This approval expands the surgical treatment options available to patients for correcting nearsightedness,” said Malvina Eydelman, M.D., director of Ophthalmic and Ear, Nose and Throat Devices, in FDA’s Center for Devices and Radiological Health.
Nearsightedness, or myopia, is a common vision condition in which close objects are seen clearly, but objects farther away are blurred. It occurs when the eye focuses light in front of the retina. This can be due to the shape of the cornea being too steep and/or the length of the eyeball being too long.
The VisuMax Femtosecond Laser removes a small amount of eye tissue to permanently reshape the cornea. A femtosecond (very fast, short-pulsed) laser makes cuts within the cornea, creating a disc-shaped piece of tissue that is removed by the surgeon through a small incision in the surface of the cornea. This tissue removal causes the shape of the cornea to change, which corrects the nearsightedness.
A clinical study of the safety and effectiveness of the device to correct nearsightedness found the procedure resulted in stable vision correction at six months. Of the 328 participants evaluated at six months, all but one had uncorrected (without glasses or contacts) visual acuity of 20/40 or better, and 88 percent had uncorrected visual acuity of 20/20 or better.
Common complications during surgery included difficulty removing the corneal tissue and loss of suction that keeps the laser aligned with your eye. Common complications after surgery included debris at the site of tissue removal, dry eye, moderate to severe glare and moderate to severe halos.
The VisuMax Femtosecond Laser is manufactured by Carl Zeiss Meditec Inc., of Dublin, Calif.