GlobeNewswire09.08.16
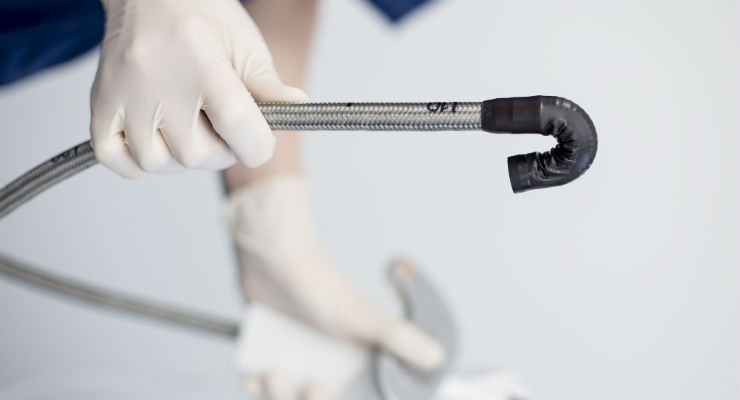
invendo medical GmbH, a developer and distributor of sterile, single-use and robotically-assisted HD endoscopy products in the field of gastroenterology and GI surgery, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the invendoscopy E200 System, which includes the invendoscope SC200—the first and only sterile, single-use colonoscope.
The invendoscope SC200 is a simple, safe, and effective solution to clinical and hygienic challenges, ensuring that a new colonoscope is always ready for physicians to use and that each patient receives his or her own device. The new advanced invendoscopy technology leads endoscopy ergonomics into the 21st century with robotic assistance for tip control and a new design to offer gastroenterologists (GIs) greater control and enhanced comfort while performing procedures.
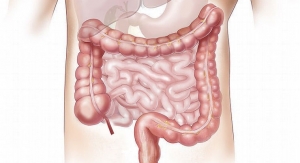
The invendoscopy E200 System has been cleared by the FDA to provide visualization and diagnostic/therapeutic access to the adult lower gastrointestinal tract (including but not limited to, the anus, rectum, sigmoid colon, colon, cecum and ileocecal valve) for endoscopy and endoscopic surgery. The colonoscope component of the invendoscopy E200 System, the invendoscope SC200, is a sterile single-use disposable device.
“Our one-of-a-kind technology provides a platform specifically tailored to address the need for device sterility during endoscopies, the importance of which has been underscored by various recent ‘superbug’ outbreaks in multiple U.S. hospitals,” said Timo Hercegfi, CEO of invendo medical. “The FDA clearance of the invendoscopy E200 System continues its pathway of validation, enabling our company to now provide endoscopists in the U.S. with a revolutionary technology that will allow them to perform colonoscopies with a system that significantly improves medical staff and patient safety while enhancing physician comfort during procedures.”
A colonoscopy is widely regarded as the gold standard for colon cancer screening. Colon cancer is the second most common cause of cancer-related deaths in the U.S.1 but may be prevented through proper screening, such as colonoscopy. The sterile, single-use invendoscope SC200 delivers a technology that improves the current colonoscopy experience by providing advanced ergonomics for the physician, enhancing safety through the elimination of risky manual cleaning processes, easing financial burden on institutions and ensuring that each patient receives a new, sterile colonoscope.
As part of the invendoscopy E200 System, the invendoscope SC200 is a sterile and single-use colonoscope that eliminates the main challenge of gastrointestinal endoscopy: the complex reprocessing of endoscopes, which is costly, overly challenging, manual-labor intensive and increases patients’ risk for cross-contamination. In addition, the invendoscope SC200 fits seamlessly into any existing clinical practice, with a low associated startup cost. The technology also improves practice efficiency by eliminating the need for scope cleaning, reprocessing, and repairs; and the invendoscope SC200’s simplified setup allows for ease of use and fast turnaround.
The CDC estimates that at least 1 in 276,000 GI procedures places a patient at risk of endoscopy-associated infection (EAI)—a six-fold increase over the initial estimate.2 More than 55 million procedures were performed with GI endoscopic devices in 2009, nearly 50 percent of them colonoscopies, and the number is increasing annually.3
Current infection control guidelines require that GI flexible endoscopes, such as colonoscopes, only be high-level disinfected, as these devices are unable to withstand the heat of high-temperature sterilization. More healthcare-associated infection outbreaks have been linked to contaminated endoscopes than to any other medical device.4 The alternative available option of prolonged gas sterilization creates a financial burden on GI practices, necessitating the purchase of additional units to ensure device availability at all times.
“In addition to the clinical benefits associated with reducing potential cross-contamination, the ergonomic design of the invendoscope SC200 offers a ScopeController that contours to the physician's hand and can be used attached or detached to the endoscope. This unique control body coupled with the lightweight of the colonoscope provides a more comfortable and less tiring procedure for the healthcare provider. The invendoscope SC200 also includes a unique tip for full retroflection in various segments of the colon, enabling inspection behind colonic folds, which is key to a comprehensive diagnosis during colonoscopies,” said John Cifarelli, Chief Commercial Officer of invendo medical.
References
1http://www.cancer.org/research/cancerfactsstatistics/
2Rutala WA, Weber DJ, and the Healthcare Control Practices Advisory Committee (HICPAC). CDC.
Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available at:http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf
3Becker's GI and Endoscopy. Available at: http://www.beckersasc.com/gastroenterology-and-endoscopy/35-statistics-about-giendoscopy-in-ascs.html
4Kenters N, Huijskens EGW, Meier C, Voss A. Infectious diseases linked to cross-contamination of flexible endoscopes. Endoscopy International Open. 2015;3(4):E259-E265. doi:10.1055/s-0034-1392099.
The invendoscope SC200 is a simple, safe, and effective solution to clinical and hygienic challenges, ensuring that a new colonoscope is always ready for physicians to use and that each patient receives his or her own device. The new advanced invendoscopy technology leads endoscopy ergonomics into the 21st century with robotic assistance for tip control and a new design to offer gastroenterologists (GIs) greater control and enhanced comfort while performing procedures.
The invendoscopy E200 System has been cleared by the FDA to provide visualization and diagnostic/therapeutic access to the adult lower gastrointestinal tract (including but not limited to, the anus, rectum, sigmoid colon, colon, cecum and ileocecal valve) for endoscopy and endoscopic surgery. The colonoscope component of the invendoscopy E200 System, the invendoscope SC200, is a sterile single-use disposable device.
“Our one-of-a-kind technology provides a platform specifically tailored to address the need for device sterility during endoscopies, the importance of which has been underscored by various recent ‘superbug’ outbreaks in multiple U.S. hospitals,” said Timo Hercegfi, CEO of invendo medical. “The FDA clearance of the invendoscopy E200 System continues its pathway of validation, enabling our company to now provide endoscopists in the U.S. with a revolutionary technology that will allow them to perform colonoscopies with a system that significantly improves medical staff and patient safety while enhancing physician comfort during procedures.”
A colonoscopy is widely regarded as the gold standard for colon cancer screening. Colon cancer is the second most common cause of cancer-related deaths in the U.S.1 but may be prevented through proper screening, such as colonoscopy. The sterile, single-use invendoscope SC200 delivers a technology that improves the current colonoscopy experience by providing advanced ergonomics for the physician, enhancing safety through the elimination of risky manual cleaning processes, easing financial burden on institutions and ensuring that each patient receives a new, sterile colonoscope.
As part of the invendoscopy E200 System, the invendoscope SC200 is a sterile and single-use colonoscope that eliminates the main challenge of gastrointestinal endoscopy: the complex reprocessing of endoscopes, which is costly, overly challenging, manual-labor intensive and increases patients’ risk for cross-contamination. In addition, the invendoscope SC200 fits seamlessly into any existing clinical practice, with a low associated startup cost. The technology also improves practice efficiency by eliminating the need for scope cleaning, reprocessing, and repairs; and the invendoscope SC200’s simplified setup allows for ease of use and fast turnaround.
The CDC estimates that at least 1 in 276,000 GI procedures places a patient at risk of endoscopy-associated infection (EAI)—a six-fold increase over the initial estimate.2 More than 55 million procedures were performed with GI endoscopic devices in 2009, nearly 50 percent of them colonoscopies, and the number is increasing annually.3
Current infection control guidelines require that GI flexible endoscopes, such as colonoscopes, only be high-level disinfected, as these devices are unable to withstand the heat of high-temperature sterilization. More healthcare-associated infection outbreaks have been linked to contaminated endoscopes than to any other medical device.4 The alternative available option of prolonged gas sterilization creates a financial burden on GI practices, necessitating the purchase of additional units to ensure device availability at all times.
“In addition to the clinical benefits associated with reducing potential cross-contamination, the ergonomic design of the invendoscope SC200 offers a ScopeController that contours to the physician's hand and can be used attached or detached to the endoscope. This unique control body coupled with the lightweight of the colonoscope provides a more comfortable and less tiring procedure for the healthcare provider. The invendoscope SC200 also includes a unique tip for full retroflection in various segments of the colon, enabling inspection behind colonic folds, which is key to a comprehensive diagnosis during colonoscopies,” said John Cifarelli, Chief Commercial Officer of invendo medical.
References
1http://www.cancer.org/research/cancerfactsstatistics/
2Rutala WA, Weber DJ, and the Healthcare Control Practices Advisory Committee (HICPAC). CDC.
Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available at:http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf
3Becker's GI and Endoscopy. Available at: http://www.beckersasc.com/gastroenterology-and-endoscopy/35-statistics-about-giendoscopy-in-ascs.html
4Kenters N, Huijskens EGW, Meier C, Voss A. Infectious diseases linked to cross-contamination of flexible endoscopes. Endoscopy International Open. 2015;3(4):E259-E265. doi:10.1055/s-0034-1392099.