Businesswire08.05.16
Capillary Biomedical Inc. has announced a research collaboration with the Sidney Kimmel Medical College of Thomas Jefferson University, supported with funding from the Juvenile Diabetes Research Foundation (JDRF), the leading global organization funding type 1 diabetes (T1D) research. The aim of this collaboration is to develop an optimized infusion catheter that will allow diabetics who use insulin pumps to receive more consistent and reliable continuous subcutaneous insulin infusion (CSII) therapy.
More than 500,000 people with diabetes in the United States on CSII pump therapy are required to change their insulin infusion catheter every two to three days. Many patients attempt to use their infusion catheters for longer durations, often resulting in poorer blood glucose control and hyperglycemia due to infusion set failure. The cause of these infusion site failures is not well understood.
Insulin pumps infuse insulin through a CSII catheter that dwells within the subcutaneous tissue for several days. The biological environment that surrounds the CSII catheter is complex and changes from hour-to-hour and day-to-day. Insulin is absorbed into the bloodstream and works to lower the blood glucose level by moving glucose (sugar) into the cells. Unfortunately, insulin absorption into the circulation can vary dose-to-dose and may become temporarily or permanently impaired on any day of catheter use.
Capillary Biomedical has licensed underlying technology from Thomas Jefferson University and, working with the school, is developing novel CSII catheters that improve the predictability of insulin dosing and enable patients to reliably use one CSII catheter for seven or more days. More consistent and reliable insulin absorption will enable people with diabetes to achieve improved blood glucose control.
In this new JDRF-funded research program, led by Jeffrey I. Joseph, D.O., professor of Anesthesiology at Sidney Medical College of Thomas Jefferson University, Capillary Biomedical will construct prototype CSII catheters and test them in a swine model for 14 days at the University. The research will visualize the flow of insulin through both commercial CSII catheters and Capillary's prototype CSII catheters into the surrounding subcutaneous tissue using micro-CT imaging. This imaging data will be correlated with histology to understand how the distribution of insulin changes over time.
This pilot data will demonstrate how well the prototype CSII catheters distribute insulin into the subcutaneous tissue every other day of wear after implantation, compared to a commercial CSII catheter. Capillary Biomedical, Inc. will then perform human clinical studies and eventually obtain regulatory approval to commercialize the novel CSII catheters and make them available to people with diabetes.
“We are excited to be working with JDRF to pursue our goal of simplifying diabetes management and to reduce its burden on both patients and the overall healthcare system,” said Paul Strasma, Capillary’s president and CEO. “Understanding and improving the predictability and speed of insulin absorption is a critical step for fully automating insulin delivery.”
“We are pleased to partner with Capillary to improve the delivery and better control of insulins,” said Aaron Kowalski, JDRF chief mission officer and vice president of Research. “The type 1 diabetes community will greatly benefit from next-generation devices that help maintain glucose control safely and conveniently. We believe advancing infusion set technology will improve those devices and the overall quality of life for people living with T1D.”
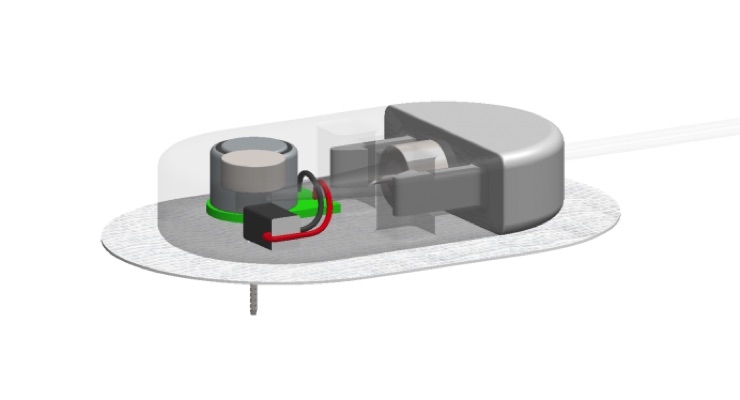
Joseph and the Capillary Biomedical team have designed a CSII catheter they hope will improve the predictability of insulin pump therapy and that will cut in half the number of required needlesticks and site changes. No stranger to innovative technology, Joseph helped found Animas Corporation and tested its infusion technology. His ultimate goal for Capillary Biomedical is to develop a long-term, fully implantable system that both senses glucose and automatically infuses the correct dose of insulin.
“The CSII catheter we will be using in this research infuses insulin from multiple small holes along the implanted catheter, rather than one large hole at the tip,” explained Joseph. “The design is somewhat similar to a soaker hose or sprinkler needle. Distributing insulin into a larger volume and surface area of subcutaneous tissue provides access to more capillary and lymph vessels, resulting in more rapid and consistent absorption and a faster mealtime insulin response with a more predictable effect on blood glucose levels.”
The Capillary CSII technology also contains a non-cutting, non-traumatic tip design and insertion method, which causes less tissue trauma, reducing inflammation and discomfort while helping to preserve precious infusion sites. Capillary Biomedical and TJU will also study the effects of CSII catheter warming and vibration on the speed and consistency of insulin absorption. It is hoped that together these features will support reliable seven-day use with a faster and more predictable insulin response.
Based in Irvine, Calif., Capillary Biomedical is a medical device startup developing technologies for diabetes management. The firm is a spin-out of the Artificial Pancreas Center at the Sidney Kimmel Medical College of Thomas Jefferson University in Philadelphia, Pa.
More than 500,000 people with diabetes in the United States on CSII pump therapy are required to change their insulin infusion catheter every two to three days. Many patients attempt to use their infusion catheters for longer durations, often resulting in poorer blood glucose control and hyperglycemia due to infusion set failure. The cause of these infusion site failures is not well understood.
Insulin pumps infuse insulin through a CSII catheter that dwells within the subcutaneous tissue for several days. The biological environment that surrounds the CSII catheter is complex and changes from hour-to-hour and day-to-day. Insulin is absorbed into the bloodstream and works to lower the blood glucose level by moving glucose (sugar) into the cells. Unfortunately, insulin absorption into the circulation can vary dose-to-dose and may become temporarily or permanently impaired on any day of catheter use.
Capillary Biomedical has licensed underlying technology from Thomas Jefferson University and, working with the school, is developing novel CSII catheters that improve the predictability of insulin dosing and enable patients to reliably use one CSII catheter for seven or more days. More consistent and reliable insulin absorption will enable people with diabetes to achieve improved blood glucose control.
In this new JDRF-funded research program, led by Jeffrey I. Joseph, D.O., professor of Anesthesiology at Sidney Medical College of Thomas Jefferson University, Capillary Biomedical will construct prototype CSII catheters and test them in a swine model for 14 days at the University. The research will visualize the flow of insulin through both commercial CSII catheters and Capillary's prototype CSII catheters into the surrounding subcutaneous tissue using micro-CT imaging. This imaging data will be correlated with histology to understand how the distribution of insulin changes over time.
This pilot data will demonstrate how well the prototype CSII catheters distribute insulin into the subcutaneous tissue every other day of wear after implantation, compared to a commercial CSII catheter. Capillary Biomedical, Inc. will then perform human clinical studies and eventually obtain regulatory approval to commercialize the novel CSII catheters and make them available to people with diabetes.
“We are excited to be working with JDRF to pursue our goal of simplifying diabetes management and to reduce its burden on both patients and the overall healthcare system,” said Paul Strasma, Capillary’s president and CEO. “Understanding and improving the predictability and speed of insulin absorption is a critical step for fully automating insulin delivery.”
“We are pleased to partner with Capillary to improve the delivery and better control of insulins,” said Aaron Kowalski, JDRF chief mission officer and vice president of Research. “The type 1 diabetes community will greatly benefit from next-generation devices that help maintain glucose control safely and conveniently. We believe advancing infusion set technology will improve those devices and the overall quality of life for people living with T1D.”
Joseph and the Capillary Biomedical team have designed a CSII catheter they hope will improve the predictability of insulin pump therapy and that will cut in half the number of required needlesticks and site changes. No stranger to innovative technology, Joseph helped found Animas Corporation and tested its infusion technology. His ultimate goal for Capillary Biomedical is to develop a long-term, fully implantable system that both senses glucose and automatically infuses the correct dose of insulin.
“The CSII catheter we will be using in this research infuses insulin from multiple small holes along the implanted catheter, rather than one large hole at the tip,” explained Joseph. “The design is somewhat similar to a soaker hose or sprinkler needle. Distributing insulin into a larger volume and surface area of subcutaneous tissue provides access to more capillary and lymph vessels, resulting in more rapid and consistent absorption and a faster mealtime insulin response with a more predictable effect on blood glucose levels.”
The Capillary CSII technology also contains a non-cutting, non-traumatic tip design and insertion method, which causes less tissue trauma, reducing inflammation and discomfort while helping to preserve precious infusion sites. Capillary Biomedical and TJU will also study the effects of CSII catheter warming and vibration on the speed and consistency of insulin absorption. It is hoped that together these features will support reliable seven-day use with a faster and more predictable insulin response.
Based in Irvine, Calif., Capillary Biomedical is a medical device startup developing technologies for diabetes management. The firm is a spin-out of the Artificial Pancreas Center at the Sidney Kimmel Medical College of Thomas Jefferson University in Philadelphia, Pa.