Business Wire06.14.16
Insulet Corporation, a provider of tubeless insulin pump technology, announced two online publications of new data in the Journal of Diabetes Science and Technology that provide evidence supporting the benefits of the Omnipod Insulin Management System in patients with type 1 and type 2 diabetes. The data show the Omnipod System is effective in controlling blood glucose levels in patients after switching from multiple daily injections (MDI) or from traditional tubed insulin pumps.
The large, multi-center retrospective study on which the publications were based showed that A1c (an important measure of blood glucose control) reduction was clinically meaningful and statistically significant for patients with type 1 diabetes at three months after starting on the Omnipod System. The study showed A1c levels decreased by 0.6 percent for patients previously on MDI therapy and were 0.5 percent lower compared to patients using traditional tubed insulin pumps. In patients with type 2 diabetes, A1c levels significantly decreased by 1.2 percent after three months of the Omnipod System use compared to prior treatment with MDI.
In addition to improvement in A1c levels, the study also showed the Omnipod System use was associated with significant reductions in daily insulin requirement and in the frequency and severity of reported hypoglycemic episodes in patients with type 1 and 2 diabetes.
“This study marks the first report of glycemic control among a large cohort of patients treated with Omnipod in a real-world clinical setting, with results comparable to those reported for multiple daily insulin injections and other continuous subcutaneous infusion pumps,” said Howard Zisser, M.D., senior author of the study. “While the patients we studied showed A1c level improvement overall, it’s significant to note the difference was greatest among those who had the least control of their condition. This suggests that poor glycemic control may be an important consideration for patients when deciding whether to initiate insulin pump therapy versus daily injections.”
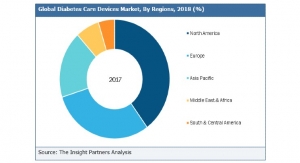
More than 900 patients were included in the study. Approximately 80 percent of the patients with type 1 diabetes were previously treated with MDI therapy and about 20 pervent used traditional insulin pumps. The group of patients with type 2 diabetes all had previously used MDI. Results at three months included:
The Omnipod Insulin Management System is a continuous insulin delivery system that provides all the proven benefits of continuous subcutaneous insulin infusion (CSII) therapy in a way no conventional insulin pump can. The Omnipod System’s innovative design and features allows people living with diabetes to live their life—and manage their diabetes—with unprecedented freedom, comfort, convenience, and ease. The Omnipod System consists of two components: (i) a Pod that stores and delivers insulin; and (ii) a Personal Diabetes Manager (PDM) that wirelessly programs the user’s personalized insulin delivery, calculates suggested doses and insulin on board, and has a convenient, built-in blood glucose meter. The small, light-weight Pod can be worn in multiple locations, including the abdomen, hip, back of upper arm, upper thigh or lower back and, because it is waterproof (IPX8), there is no need to remove when showering, swimming or performing other activities.
This means that Omnipod can provide up to three days of non-stop insulin delivery, without the need to disconnect a tube set or manually inject insulin. The Pod and PDM communicate wirelessly to offer precise, personalized and continuous insulin delivery with customizable basal and bolus delivery options, as well as important safety checks. The Pod’s auto-cannula insertion is quick, simple, and virtually pain-free. Users never have to handle a needle. The user simply pushes a button on the PDM and the Pod's automated insertion system inserts the cannula beneath the skin and begins delivering insulin according to the user's programmed basal rate.
The Omnipod System is touted as the world’s first commercially available tubeless insulin delivery system that allows users to live untethered by tubing and without the stress and anxiety of multiple daily injections. By breaking down the barriers to insulin pump therapy, the Omnipod System offers freedom for users to live life on their own terms and with the ease of use they deserve.
Insulet Corporation seeks to expand the use of insulin pump therapy among people with insulin-dependent diabetes. The Omnipod is a revolutionary and easy-to-use tubeless insulin pump that features just two parts and a fully-automated cannula insertion. Insulet’s Delivery Systems business also partners with global pharmaceutical and biotechnology companies to tailor the Omnipod technology platform for the delivery of subcutaneous drugs across multiple therapeutic areas. Founded in 2000, Insulet is based in Billerica, Mass.
The large, multi-center retrospective study on which the publications were based showed that A1c (an important measure of blood glucose control) reduction was clinically meaningful and statistically significant for patients with type 1 diabetes at three months after starting on the Omnipod System. The study showed A1c levels decreased by 0.6 percent for patients previously on MDI therapy and were 0.5 percent lower compared to patients using traditional tubed insulin pumps. In patients with type 2 diabetes, A1c levels significantly decreased by 1.2 percent after three months of the Omnipod System use compared to prior treatment with MDI.
In addition to improvement in A1c levels, the study also showed the Omnipod System use was associated with significant reductions in daily insulin requirement and in the frequency and severity of reported hypoglycemic episodes in patients with type 1 and 2 diabetes.
“This study marks the first report of glycemic control among a large cohort of patients treated with Omnipod in a real-world clinical setting, with results comparable to those reported for multiple daily insulin injections and other continuous subcutaneous infusion pumps,” said Howard Zisser, M.D., senior author of the study. “While the patients we studied showed A1c level improvement overall, it’s significant to note the difference was greatest among those who had the least control of their condition. This suggests that poor glycemic control may be an important consideration for patients when deciding whether to initiate insulin pump therapy versus daily injections.”
More than 900 patients were included in the study. Approximately 80 percent of the patients with type 1 diabetes were previously treated with MDI therapy and about 20 pervent used traditional insulin pumps. The group of patients with type 2 diabetes all had previously used MDI. Results at three months included:
- Improved A1c levels: Type 1 diabetes patients, including the pediatric, adolescent and adult groups, showed significant (p<0.001) decreases in A1c of 0.6 percent and 0.5 percent with the Omnipod System compared to MDI and traditional tubed pumps, respectively. Patients with type 2 diabetes using the Omnipod System had a 1.2 percent decrease in A1c compared to MDI.
- Reduced daily insulin requirement: A 16.4 percent reduction in the total daily dose of insulin was observed in patients with type 1 diabetes using the Omnipod System and a 27.5 percent reduction was reported for patients with type 2 diabetes.
-
Fewer hypoglycemic episodes: Patients with type 1 diabetes reported that hypoglycemia decreased significantly by one episode per week (p<0.001) with the Omnipod System treatment compared to previous treatments and that the severity of hypoglycemic episodes was also significantly lower. In patients with type 2 diabetes, the frequency of hypoglycemic episodes was 46.2 percent (p<0.004) lower with the Omnipod System treatment, with similar reductions in severity of hypoglycemic episodes.
The Omnipod Insulin Management System is a continuous insulin delivery system that provides all the proven benefits of continuous subcutaneous insulin infusion (CSII) therapy in a way no conventional insulin pump can. The Omnipod System’s innovative design and features allows people living with diabetes to live their life—and manage their diabetes—with unprecedented freedom, comfort, convenience, and ease. The Omnipod System consists of two components: (i) a Pod that stores and delivers insulin; and (ii) a Personal Diabetes Manager (PDM) that wirelessly programs the user’s personalized insulin delivery, calculates suggested doses and insulin on board, and has a convenient, built-in blood glucose meter. The small, light-weight Pod can be worn in multiple locations, including the abdomen, hip, back of upper arm, upper thigh or lower back and, because it is waterproof (IPX8), there is no need to remove when showering, swimming or performing other activities.
This means that Omnipod can provide up to three days of non-stop insulin delivery, without the need to disconnect a tube set or manually inject insulin. The Pod and PDM communicate wirelessly to offer precise, personalized and continuous insulin delivery with customizable basal and bolus delivery options, as well as important safety checks. The Pod’s auto-cannula insertion is quick, simple, and virtually pain-free. Users never have to handle a needle. The user simply pushes a button on the PDM and the Pod's automated insertion system inserts the cannula beneath the skin and begins delivering insulin according to the user's programmed basal rate.
The Omnipod System is touted as the world’s first commercially available tubeless insulin delivery system that allows users to live untethered by tubing and without the stress and anxiety of multiple daily injections. By breaking down the barriers to insulin pump therapy, the Omnipod System offers freedom for users to live life on their own terms and with the ease of use they deserve.
Insulet Corporation seeks to expand the use of insulin pump therapy among people with insulin-dependent diabetes. The Omnipod is a revolutionary and easy-to-use tubeless insulin pump that features just two parts and a fully-automated cannula insertion. Insulet’s Delivery Systems business also partners with global pharmaceutical and biotechnology companies to tailor the Omnipod technology platform for the delivery of subcutaneous drugs across multiple therapeutic areas. Founded in 2000, Insulet is based in Billerica, Mass.