Business Wire05.19.16
New clinical data from two high-quality randomized studies of NeoTract Inc.’s UroLift System for patients with benign prostatic hyperplasia (BPH) show the device quickly reduces symptoms and preserves sexual function.
The conclusions were drawn from four-year follow-up data from the pivotal, randomized L.I.F.T. IDE study, which evaluated the safety and effectiveness of the UroLift System in patients with symptomatic BPH, and two-year data from the BPH-6 study, which compared the UroLift System with the surgical standard, transurethral resection of the prostate (TURP).
“The clinical data show that treatment with the UroLift system offered men suffering from BPH a well-tolerated minimally invasive experience that provided uniquely rapid relief and a durable, sustained effect,” said Dave Amerson, president and CEO, NeoTract. “We are proud to offer this treatment option that can allow men to stop taking BPH medications and be treated with a simple procedure that does not carry the risk of sexual side effects common with other treatment options.”
Data from the L.I.F.T. IDE pivotal study demonstrated durable improvement in symptoms, quality of life and urinary flow at four years.
“While prior data have shown that this treatment option offers rapid relief with low morbidity, the four-year L.I.F.T. study data demonstrate solid durability for a less invasive treatment option,” said Dr. Claus Roehrborn, co-principal investigator for the L.I.F.T. clinical program and professor/chairman of the Department of Urology UT Southwester Medical Center in Dallas, Texas. “After four years, over 86 percent of patients were free from seeking additional procedural treatment for BPH.”
The L.I.F.T. IDE Study enrolled 206 patients in a multi-center, randomized, blinded study to evaluate the safety and effectiveness of the UroLift System in patients with symptomatic BPH.
The four-year L.I.F.T. study analysis demonstrated that UroLift system treatment provided:
The BPH-6 post-market study is the first-ever randomized comparison between UroLift and TURP, the most common surgical treatment for BPH. The two-year data demonstrated that the UroLift device continued to respond more consistently than TURP when assessed for overall clinical improvement. This composite BPH6 endpoint defines overall clinical improvement as improved lower urinary tract symptoms (LUTS) without diminished sexual function, continence or safety.
“While TURP is the most common treatment for symptoms caused by an enlarged prostate, the side effects can be significant and may cause men to postpone or refuse treatment altogether,” said Jens Sønksen, M.D., president of the Danish Association of Urology. “The results of this study provided an overall assessment of the ability of UroLift to improve symptoms without compromising important aspects of health, such as sexual function. We were also delighted to see that UroLift demonstrated superior quality of recovery. These characteristics are of great importance to patients when deciding how to address their symptoms.”
The BPH-6 study, a prospective, randomized study, compared the UroLift system treatment to TURP in 80 patients at 10 European centers, with a goal of evaluating the ability of the UroLift System to improve a patient's overall clinical improvement as compared to TURP.
Additional key data points from the study, at two years post-treatment:
More than 500 million men globally suffer from BPH, a condition in which the prostate enlarges as men get older. Chronic lower urinary tract symptoms (LUTS) associated with BPH can cause loss of productivity and sleep, depression and decreased quality of life. Medication is often the first line therapy but relief can be inadequate and temporary. Side-effects can include sexual dysfunction, dizziness and headaches, prompting many patients to quit using the drugs. For these patients, the classic alternative has been surgery that cuts or heats prostate tissue to open the blocked urethra. Although effective, patients typically only achieve symptom relief after a difficult period of irritating voiding symptoms and wearing a urinary catheter. Even the ‘gold standard’ surgery, TURP (Transurethral Resection of the Prostate), can leave patients with permanent side effects such as urinary incontinence, erectile dysfunction and retrograde ejaculation (dry orgasm).
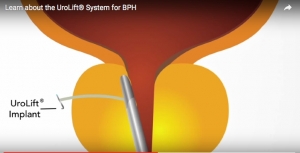
The U.S. Food and Drug Administration-cleared UroLift System is a minimally invasive technology for treating lower urinary tract symptoms due to benign prostatic hyperplasia. The UroLift permanent implants, delivered during a minimally invasive transurethral outpatient procedure, relieve prostate obstruction and open the urethra directly without cutting, heating, or removing prostate tissue. The UroLift System is available in the United States, Europe, Australia, Mexico, South Korea and Canada.
Pleasanton, Calif.-based NeoTract Inc. develops minimally invasive devices that address unmet needs in the field of urology.
The conclusions were drawn from four-year follow-up data from the pivotal, randomized L.I.F.T. IDE study, which evaluated the safety and effectiveness of the UroLift System in patients with symptomatic BPH, and two-year data from the BPH-6 study, which compared the UroLift System with the surgical standard, transurethral resection of the prostate (TURP).
“The clinical data show that treatment with the UroLift system offered men suffering from BPH a well-tolerated minimally invasive experience that provided uniquely rapid relief and a durable, sustained effect,” said Dave Amerson, president and CEO, NeoTract. “We are proud to offer this treatment option that can allow men to stop taking BPH medications and be treated with a simple procedure that does not carry the risk of sexual side effects common with other treatment options.”
Data from the L.I.F.T. IDE pivotal study demonstrated durable improvement in symptoms, quality of life and urinary flow at four years.
“While prior data have shown that this treatment option offers rapid relief with low morbidity, the four-year L.I.F.T. study data demonstrate solid durability for a less invasive treatment option,” said Dr. Claus Roehrborn, co-principal investigator for the L.I.F.T. clinical program and professor/chairman of the Department of Urology UT Southwester Medical Center in Dallas, Texas. “After four years, over 86 percent of patients were free from seeking additional procedural treatment for BPH.”
The L.I.F.T. IDE Study enrolled 206 patients in a multi-center, randomized, blinded study to evaluate the safety and effectiveness of the UroLift System in patients with symptomatic BPH.
The four-year L.I.F.T. study analysis demonstrated that UroLift system treatment provided:
- Rapid reduction of symptoms with low morbidity, while preserving sexual function;
- Sustained effect, with IPSS (International Prostate Symptom Score) and Qmax (peak urinary flow rate) remaining 41 percent and 62 percent improved from baseline, respectively;
- An improved quality of life (QoL), with the QoL score improved 52 percent over four years; and
- Freedom from retreatment for most patients, with 9 percent requiring alternative treatment and 4 percent requiring additional UroLift implants over the course of four years.
The BPH-6 post-market study is the first-ever randomized comparison between UroLift and TURP, the most common surgical treatment for BPH. The two-year data demonstrated that the UroLift device continued to respond more consistently than TURP when assessed for overall clinical improvement. This composite BPH6 endpoint defines overall clinical improvement as improved lower urinary tract symptoms (LUTS) without diminished sexual function, continence or safety.
“While TURP is the most common treatment for symptoms caused by an enlarged prostate, the side effects can be significant and may cause men to postpone or refuse treatment altogether,” said Jens Sønksen, M.D., president of the Danish Association of Urology. “The results of this study provided an overall assessment of the ability of UroLift to improve symptoms without compromising important aspects of health, such as sexual function. We were also delighted to see that UroLift demonstrated superior quality of recovery. These characteristics are of great importance to patients when deciding how to address their symptoms.”
The BPH-6 study, a prospective, randomized study, compared the UroLift system treatment to TURP in 80 patients at 10 European centers, with a goal of evaluating the ability of the UroLift System to improve a patient's overall clinical improvement as compared to TURP.
Additional key data points from the study, at two years post-treatment:
- UroLift provided superior quality of recovery and preservation of sexual function as compared to TURP, p<0.01.
- Both UroLift and TURP provided significant symptom relief, quality of life and flow improvement through two years.
- While improvement in International IPSS and Qmax were statistically superior for TURP, this did not result in a difference in quality of life improvement.
- The need for additional treatment, whether for LUTS or complications, was the same between groups, 14 percent over two years.
More than 500 million men globally suffer from BPH, a condition in which the prostate enlarges as men get older. Chronic lower urinary tract symptoms (LUTS) associated with BPH can cause loss of productivity and sleep, depression and decreased quality of life. Medication is often the first line therapy but relief can be inadequate and temporary. Side-effects can include sexual dysfunction, dizziness and headaches, prompting many patients to quit using the drugs. For these patients, the classic alternative has been surgery that cuts or heats prostate tissue to open the blocked urethra. Although effective, patients typically only achieve symptom relief after a difficult period of irritating voiding symptoms and wearing a urinary catheter. Even the ‘gold standard’ surgery, TURP (Transurethral Resection of the Prostate), can leave patients with permanent side effects such as urinary incontinence, erectile dysfunction and retrograde ejaculation (dry orgasm).
The U.S. Food and Drug Administration-cleared UroLift System is a minimally invasive technology for treating lower urinary tract symptoms due to benign prostatic hyperplasia. The UroLift permanent implants, delivered during a minimally invasive transurethral outpatient procedure, relieve prostate obstruction and open the urethra directly without cutting, heating, or removing prostate tissue. The UroLift System is available in the United States, Europe, Australia, Mexico, South Korea and Canada.
Pleasanton, Calif.-based NeoTract Inc. develops minimally invasive devices that address unmet needs in the field of urology.