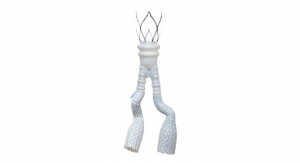
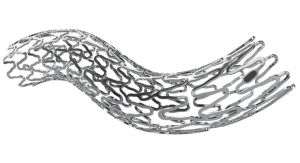
Micell Technologies is releasing its flagship product, the MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System, in the European market. The stent is designed to release rapamycin to prevent restenosis, but unlike similar stents the crystalline drug stays within the nearby tissue for months after the coating and polymer surrounding the cobalt chromium metal stent have been absorbed.
The technology relies on Micell’s patented supercritical fluid technology that allows the drug together with the polymer to stick to the metal stent. The underlying stent is actually the Genius MAGIC made by Eurocor that features a particularly thin strut design.
“Patients with cardiovascular disease and their physicians now have access to a new class of drug-eluting stent that allows the drug to remain in the tissue for an extended period -- well beyond a rapidly-absorbing polymer," said Arthur J. Benvenuto, chairman/CEO of Micell. "This performance feature is unique to MiStent and until now, has been largely impossible. Most importantly, it has translated into meaningful clinical benefits.”
MiStent SES has received CE Mark in the European Union, and will be distributed exclusively by Stentys S.A. everywhere but the United States, Canada, China, South Korea and Japan. Micell currently is attempting to expand its manufacturing capabilities through its partner, Surgical Technologies Inc., based in St. Paul, Minn.
“We are very excited to be partnering with Micell on the distribution of MiStent,” said Gonzague Issenmann, CEO of Stentys. “We believe MiStent is a great addition to our stent portfolio, and we look forward to launching this innovative product.”
MiStent SES is designed to optimize healing in patients with coronary artery disease. The rapidly absorbable coating of the MiStent SES is intended to precisely and consistently provide for local drug delivery and limit the duration of polymer exposure, thereby potentially reducing the safety risks associated with current commercially available drug-eluting stents, Micell claims. The MiStent SES includes a proprietary stent coating that contains crystalline drug (sirolimus) and an absorbable polymer. The coating provides controlled and sustained release of therapeutic levels of drug as the polymer disperses from the stent into the adjacent tissue. These properties are intended to enhance safety as compared to conventional permanent polymer drug-eluting stents. Results of animal studies have determined that the coating is cleared from the stent in 45 to 60 days, leaving a bare-metal stent, and the polymer is completely absorbed into the surrounding tissue within 90 days to promote long-term patency and compatibility with the artery.
The crystalline drug remains in the tissue and continues to elute sirolimus for up to nine months. Using an approved drug (sirolimus) and polymer (PLGA), Micell's patented supercritical fluid technology allows a rigorously controlled drug/polymer coating to be applied to a bare-metal stent. The MiStent SES leverages the benefits of a cobalt chromium coronary stent system, a thin-strut, bare-metal stent, which has demonstrated excellent deliverability, conformability and flexibility, Micell bigwigs noted. EU approval of MiStent SES was supported by clinical data from two studies, DESSOLVE I and II, which demonstrated superior in-stent late lumen loss rates and an excellent safety profile. The three-year follow-up of the DESSOLVE clinical studies subjects was completed in 2014, and these patients continue to undergo long-term followup.
Based in Paris, France, Stentys develops and commercializes complex coronary artery disease treatment. The company's Self-Apposing stents are designed to adapt to vessels with ambiguous or fluctuating diameters to prevent the malapposition problems associated with conventional stents.
Micell Technologies is a Durham, N.C.-based biomedical company developing interventional cardiology systems for vascular drug delivery.