FDA Grants Three Emergency Use Authorizations for COVID-19 Rapid Antigen Tests
By Globe Newswire | 08.18.21
OraSure Technologies' tests are authorized for over-the-counter use without a prescription, as well as for prescription and professional use in POC settings.
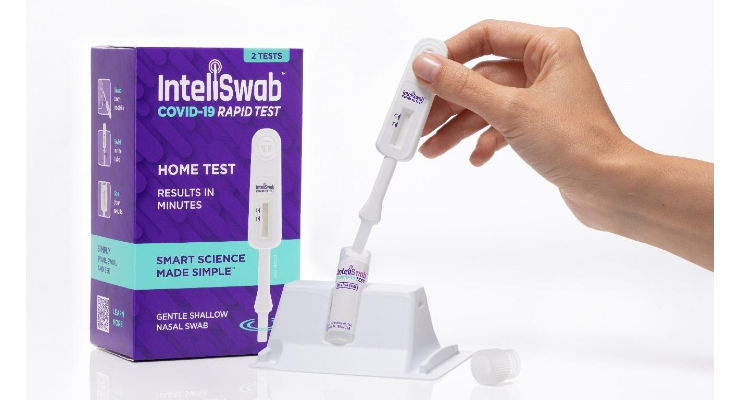
OraSure Technologies Inc. has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its COVID-19 rapid antigen tests, which the company is branding as InteliSwab. These tests detect active COVID-19 infection. The FDA has authorized the InteliSwab COVID-19 Rapid Test for Over-the-Counter (OTC) use without a prescription. FDA has also authorized the InteliSwab COVID-19 Rapid Test Pro for professional use in point of care (POC) CLIA-waived settings, and the InteliSwab COVID-19 Rapid Test Rx for Prescription Home Use.
OraSure will market three versions of InteliSwab:
The design of InteliSwab incorporates a built-in swab that is fully integrated into the test stick, simplifying the entire testing process. Use of this integrated swab also helps ensure supply continuity, as InteliSwab does not require sourcing scarce nasal swabs. Testing with InteliSwab is simple: Users swab their lower nostrils with the test stick, swirl it in a pre-measured solution, and see their result on the test stick in 30 minutes—with no instruments, batteries, smartphone or laboratory analysis needed to see the result. Using InteliSwab to test for COVID-19 requires less than one minute of “hands-on” time.
In a clinical study at five independent U.S. sites, InteliSwab had strong performance, with positive results agreeing with highly sensitive FDA-authorized PCR tests 84 percent of the time, and negative results agreeing 98 percent of the time. In addition, 98 percent of consumers in the clinical trial found InteliSwab easy-to-use.
“OraSure is on a mission to make COVID-19 testing dramatically simpler. We believe that this easy and intuitive ‘swab, swirl and see’ test will be one of the simplest COVID-19 tests on the market. We expect that InteliSwab’s simplicity and accuracy will give users peace of mind that they performed the test correctly and can rely on the results,” said OraSure President and CEO Stephen Tang, Ph.D. “Simple and accessible at-home tests, like InteliSwab, make it easier for individuals to know if they are infectious and to quickly self-isolate if they test positive. With InteliSwab, we believe OraSure will play an even larger role in safely reopening—and keeping open—workplaces, schools and other places where people congregate.”
The company’s installed manufacturing capacity for InteliSwab is currently 55 million units annually; the company is ramping up to a production capacity of 70 million units annually in the third quarter.
OraSure will be completing the required post market studies as specified in the FDA authorization letter.
OraSure will market three versions of InteliSwab:
- InteliSwab COVID-19 Rapid Test: The OTC home test for use without a prescription in individuals 15 years or older (with or without symptoms) when tested twice over two or three days with at least 24 and no more than 36 hours between tests.
- InteliSwab COVID-19 Rapid Test Pro: The professional test, which is packaged in bulk configurations, is authorized for use at the point of care in healthcare settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation, for individuals 15 years or older who are suspected of COVID-19 by their healthcare provider within seven days of symptom onset or for individuals without symptoms when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests.
- InteliSwab COVID-19 Rapid Test Rx: The prescription home test is authorized for prescription home use with self-collection (unobserved) of anterior nasal samples from individuals 18 years or older or adult collected samples from individuals age 15 years or older who are suspected of COVID-19 infection by their healthcare provider within the first seven days of symptom onset.
The design of InteliSwab incorporates a built-in swab that is fully integrated into the test stick, simplifying the entire testing process. Use of this integrated swab also helps ensure supply continuity, as InteliSwab does not require sourcing scarce nasal swabs. Testing with InteliSwab is simple: Users swab their lower nostrils with the test stick, swirl it in a pre-measured solution, and see their result on the test stick in 30 minutes—with no instruments, batteries, smartphone or laboratory analysis needed to see the result. Using InteliSwab to test for COVID-19 requires less than one minute of “hands-on” time.
In a clinical study at five independent U.S. sites, InteliSwab had strong performance, with positive results agreeing with highly sensitive FDA-authorized PCR tests 84 percent of the time, and negative results agreeing 98 percent of the time. In addition, 98 percent of consumers in the clinical trial found InteliSwab easy-to-use.
“OraSure is on a mission to make COVID-19 testing dramatically simpler. We believe that this easy and intuitive ‘swab, swirl and see’ test will be one of the simplest COVID-19 tests on the market. We expect that InteliSwab’s simplicity and accuracy will give users peace of mind that they performed the test correctly and can rely on the results,” said OraSure President and CEO Stephen Tang, Ph.D. “Simple and accessible at-home tests, like InteliSwab, make it easier for individuals to know if they are infectious and to quickly self-isolate if they test positive. With InteliSwab, we believe OraSure will play an even larger role in safely reopening—and keeping open—workplaces, schools and other places where people congregate.”
The company’s installed manufacturing capacity for InteliSwab is currently 55 million units annually; the company is ramping up to a production capacity of 70 million units annually in the third quarter.
OraSure will be completing the required post market studies as specified in the FDA authorization letter.