How COVID-19 is Reshaping the Diagnostics Testing Sector
By Eric Williams, Managing Director, Capstone Headwaters | 11.04.20
The short-term impact on the diagnostics sector is readily apparent as the pandemic has fueled overwhelming demand for effective SARS-CoV-2 testing solutions.
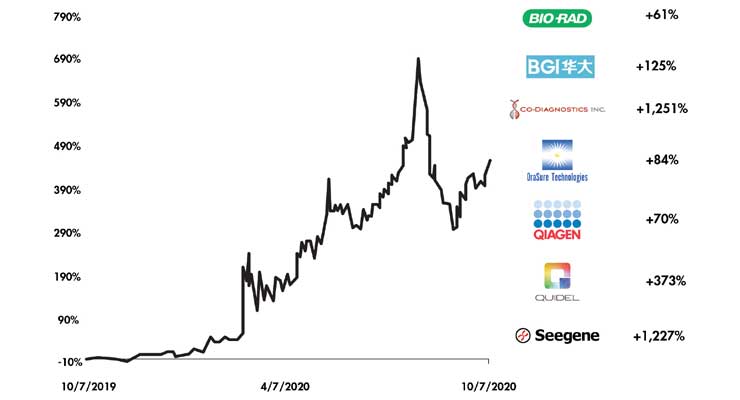
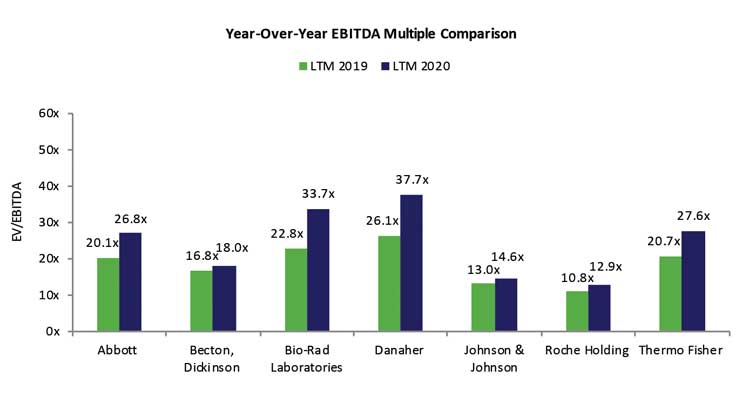
The short-term impact on the diagnostics sector is readily apparent as the pandemic has fueled overwhelming demand for effective and rapid SARS-CoV-2 testing solutions. Testing represents a critical means to gauge and contain COVID-19’s spread, and estimates suggest approximately 30 million tests are needed weekly in the U.S. alone, according to The Rockefeller Foundation.1 The market has rewarded players that have bolstered testing capacity and developed innovative technology to support the fight against COVID-19. Valuations for a composite small to mid-sized player are up four-fold in the past 12 months while the likes of Abbott, Roche, BD, and Thermo Fisher Scientific are up an average of 30 percent. In addition, top public players including Quidel and QIAGEN have experienced year over year EBITDA multiple expansion of 75.1 percent and 41.2 percent, respectively.
With the upcoming double threat of flu and COVID-19, a second wave hitting Europe, and the U.S. continuing to record 40,000-50,000 new daily cases, boosting testing capacity is paramount. Diagnostics players have sought to rapidly expand manufacturing capacity, presenting attractive opportunities for sophisticated contract manufacturing organizations (CMOs) to assist in scaling production. With more than 100 million cases of potential flu-like symptoms in the U.S. alone, it is critically important to rapidly differentiate between the pathogens for proper treatment and spread prevention. The application of diagnostic testing, specifically point-of-care, will be critical for enhancing disease detection, decision making and contagion containment. Hospitals’ point-of-care test use will be vital this flu season and perhaps for years to come to differentiate between influenza and SARS-CoV-2. Proper diagnosis is critical as hospitals attempt to avoid misdiagnosis and overcrowding, which threatens lives and overwhelms hospital capacity.
Efforts to expand testing domestically and internationally have been hampered by supply challenges in securing reagents and even laboratory plastic consumables such as pipette and automation tips, storage tubes and plates, transfer pipettes, and packaging vials and bottles. Approximately 49 percent of laboratories alluded to supply sourcing as the biggest challenge during the pandemic, according to Medical Laboratory Observer.2 Notably, Quidel Corporation has had trouble procuring nasal foam swabs due to variable supply levels.3 Thermo Fisher Scientific is investing more than $140 million to expand production of its laboratory plastics consumables to meet global demand for COVID-19 testing and vaccine development/manufacturing.4 The company plans to add 1,000 jobs across its global manufacturing sites to address the need for applications including laboratory plastics, pipette and automation tips, storage tubes, and packaging vials and bottles. While reagent shortages have led to testing delays, it has also spurred innovation in the sector. For example, a number of companies are developing COVID-19 RNA extraction-less tests, which eliminates the need for expensive RNA extraction reagents as well as pipette and automation tips.
CMOs have proved invaluable in efficiently scaling production. Notably, Co-Diagnostics has contracted Promega to provide additional manufacturing capacity for its Logix Smart COVID-19 test as it seeks to expand its outreach in public and private laboratories.5 Longhorn Vaccines and Diagnostics, which has developed the only available FDA-cleared microbial nucleic acid storage and stabilization device (PrimeStore MTM) to inactivate the coronavirus, has leveraged strong relationships with contract manufacturers such as EKF Diagnostics to meet unprecedented demand for its sample collection product.
“We were eager to equip laboratories and point-of-care providers with PrimeStore MTM since it reduces the risk of infection to individuals involved in the collection, transfer, and testing processes,” Jeff Fischer, president of Longhorn Vaccines and Diagnostics, commented in a press release. “The device also stabilizes and preserves RNA at ambient temperatures eliminating the need for cold storage transport, simplifying lab procedures, and thus facilitating more reliable and accurate testing.” Longhorn Vaccines and Diagnostics recently launched PrimeStore ATM, an extractionless PCR solution that eliminates the need for extraction components.6
Public Company Update
Public companies in the Diagnostic Testing industry have capitalized on newfound demand for effective testing solutions, capturing unprecedented levels of revenue growth. Notably, Co-Diagnostics recorded a 1,503 percent revenue increase in Q2 due largely to sales of its Logix Smart COVID-19 test. Likewise, sales of Novacyt’s PCR COVID-19 test has contributed to a 900 percent revenue spike year-over-year. Other notable industry participants including QIAGEN and Quidel have recorded revenue increases of 16.2 percent and 86.4 percent, respectively.
Companies have also used M&A to bolster their diagnostic platforms and expand into new markets. In September, QIAGEN acquired the remaining 80.1 percent stake in diagnostics instruments company NeuMoDx Molecular for $309.6 million. NeuMoDx is a molecular diagnostics solutions provider offering rapid, integrated PCR-based devices that provide assays for various infectious diseases. The company also developed a COVID-19 test that received FDA Emergency Use Authorization in late March. The deal highlights suitors’ appetite for technology that enables more rapid results, and automation that simplifies processes and increases testing throughput. “NeuMoDx’s automated molecular testing platforms offer a combination of speed, flexibility, throughput, and ease-of-use for molecular diagnostics assays, including laboratory-developed tests,” QIAGEN CEO Thierry Bernard said in announcing the transaction. “NeuMoDx has built a platform that has demonstrated superior value during the coronavirus pandemic. This will expand QIAGEN’s portfolio of automated testing solutions and provide another driver for future growth.”
While COVID-19-related solutions are currently a priority, the pandemic has also highlighted the need for better surveillance tools for novel pathogens and improved methods for battling other infectious diseases. “The U.S. has been spared from the wrath of drug-resistant TB so far, but may no longer be that lucky. We need to prepare. We may start seeing strains that are atypical in the United States very soon,“ warned Faramarz Valafar, a professor at San Diego State University’s School of Public Health and a NIAID principal investigator.
No one knows how long the pandemic will last, but many experts anticipate the coronavirus battle to extend well beyond 2021. “We may never eradicate the virus but simply control it via public health measures and medicine,” Dr. Anthony Fauci said. While vaccine development efforts have progressed at unprecedented speeds, there remains significant manufacturing challenges to large-scale production. In addition, a substantial proportion of the public is skeptical about a vaccination—approximately 35 percent of Americans unwilling to receive a potential COVID-19 vaccine, according to Gallup.7 This reluctance is not just a U.S. phenomenon, as a University of Hamburg poll indicated that nearly 40 percent of Germans were unwilling to be vaccinated. Those amenable to a vaccination may have to wait due to manufacturing constraints, which could be exacerbated if multiple doses are required. Adar Poonawlaa, CEO of the Serum Institute of India (the world’s largest vaccine manufacturer) said it would take four to five years to vaccinate everyone on the planet.8 More disconcerting was a recent comment made by David Morens, virologist at the National Institute of Allergy and Infectious Diseases and senior advisor to Fauci, director of the NIAID: “Although we don’t know yet, it is well within the realm of possibility that this coronavirus, when our population-level immunity gets high enough, will find a way to get around our immunity. If that happened, we’d be in the same situation as with flu. We’ll have to chase the virus and, as it mutates, we’ll have to tinker with our vaccine.”9
References
Eric Williams leads Capstone’s Health and Medical practice. Previously, he managed the Eastern region for Morgan Stanley Smith Barney’s Capital Strategies Group and its predecessor, Citi Capital Strategies. Eric has more than 20 years of experience managing strategic sale assignments and leveraged recapitalizations for owners of privately held companies. He has successfully completed over 100 transactions in a various subsectors including medical device, diagnostics, contract manufacturing, and pharmaceutical outsourcing. He is a Series 7 and 63 Registered Securities Representative as well as a Series 24 Registered Securities Principal.
With the upcoming double threat of flu and COVID-19, a second wave hitting Europe, and the U.S. continuing to record 40,000-50,000 new daily cases, boosting testing capacity is paramount. Diagnostics players have sought to rapidly expand manufacturing capacity, presenting attractive opportunities for sophisticated contract manufacturing organizations (CMOs) to assist in scaling production. With more than 100 million cases of potential flu-like symptoms in the U.S. alone, it is critically important to rapidly differentiate between the pathogens for proper treatment and spread prevention. The application of diagnostic testing, specifically point-of-care, will be critical for enhancing disease detection, decision making and contagion containment. Hospitals’ point-of-care test use will be vital this flu season and perhaps for years to come to differentiate between influenza and SARS-CoV-2. Proper diagnosis is critical as hospitals attempt to avoid misdiagnosis and overcrowding, which threatens lives and overwhelms hospital capacity.
Efforts to expand testing domestically and internationally have been hampered by supply challenges in securing reagents and even laboratory plastic consumables such as pipette and automation tips, storage tubes and plates, transfer pipettes, and packaging vials and bottles. Approximately 49 percent of laboratories alluded to supply sourcing as the biggest challenge during the pandemic, according to Medical Laboratory Observer.2 Notably, Quidel Corporation has had trouble procuring nasal foam swabs due to variable supply levels.3 Thermo Fisher Scientific is investing more than $140 million to expand production of its laboratory plastics consumables to meet global demand for COVID-19 testing and vaccine development/manufacturing.4 The company plans to add 1,000 jobs across its global manufacturing sites to address the need for applications including laboratory plastics, pipette and automation tips, storage tubes, and packaging vials and bottles. While reagent shortages have led to testing delays, it has also spurred innovation in the sector. For example, a number of companies are developing COVID-19 RNA extraction-less tests, which eliminates the need for expensive RNA extraction reagents as well as pipette and automation tips.
CMOs have proved invaluable in efficiently scaling production. Notably, Co-Diagnostics has contracted Promega to provide additional manufacturing capacity for its Logix Smart COVID-19 test as it seeks to expand its outreach in public and private laboratories.5 Longhorn Vaccines and Diagnostics, which has developed the only available FDA-cleared microbial nucleic acid storage and stabilization device (PrimeStore MTM) to inactivate the coronavirus, has leveraged strong relationships with contract manufacturers such as EKF Diagnostics to meet unprecedented demand for its sample collection product.
“We were eager to equip laboratories and point-of-care providers with PrimeStore MTM since it reduces the risk of infection to individuals involved in the collection, transfer, and testing processes,” Jeff Fischer, president of Longhorn Vaccines and Diagnostics, commented in a press release. “The device also stabilizes and preserves RNA at ambient temperatures eliminating the need for cold storage transport, simplifying lab procedures, and thus facilitating more reliable and accurate testing.” Longhorn Vaccines and Diagnostics recently launched PrimeStore ATM, an extractionless PCR solution that eliminates the need for extraction components.6
Public Company Update
Public companies in the Diagnostic Testing industry have capitalized on newfound demand for effective testing solutions, capturing unprecedented levels of revenue growth. Notably, Co-Diagnostics recorded a 1,503 percent revenue increase in Q2 due largely to sales of its Logix Smart COVID-19 test. Likewise, sales of Novacyt’s PCR COVID-19 test has contributed to a 900 percent revenue spike year-over-year. Other notable industry participants including QIAGEN and Quidel have recorded revenue increases of 16.2 percent and 86.4 percent, respectively.
Companies have also used M&A to bolster their diagnostic platforms and expand into new markets. In September, QIAGEN acquired the remaining 80.1 percent stake in diagnostics instruments company NeuMoDx Molecular for $309.6 million. NeuMoDx is a molecular diagnostics solutions provider offering rapid, integrated PCR-based devices that provide assays for various infectious diseases. The company also developed a COVID-19 test that received FDA Emergency Use Authorization in late March. The deal highlights suitors’ appetite for technology that enables more rapid results, and automation that simplifies processes and increases testing throughput. “NeuMoDx’s automated molecular testing platforms offer a combination of speed, flexibility, throughput, and ease-of-use for molecular diagnostics assays, including laboratory-developed tests,” QIAGEN CEO Thierry Bernard said in announcing the transaction. “NeuMoDx has built a platform that has demonstrated superior value during the coronavirus pandemic. This will expand QIAGEN’s portfolio of automated testing solutions and provide another driver for future growth.”
While COVID-19-related solutions are currently a priority, the pandemic has also highlighted the need for better surveillance tools for novel pathogens and improved methods for battling other infectious diseases. “The U.S. has been spared from the wrath of drug-resistant TB so far, but may no longer be that lucky. We need to prepare. We may start seeing strains that are atypical in the United States very soon,“ warned Faramarz Valafar, a professor at San Diego State University’s School of Public Health and a NIAID principal investigator.
No one knows how long the pandemic will last, but many experts anticipate the coronavirus battle to extend well beyond 2021. “We may never eradicate the virus but simply control it via public health measures and medicine,” Dr. Anthony Fauci said. While vaccine development efforts have progressed at unprecedented speeds, there remains significant manufacturing challenges to large-scale production. In addition, a substantial proportion of the public is skeptical about a vaccination—approximately 35 percent of Americans unwilling to receive a potential COVID-19 vaccine, according to Gallup.7 This reluctance is not just a U.S. phenomenon, as a University of Hamburg poll indicated that nearly 40 percent of Germans were unwilling to be vaccinated. Those amenable to a vaccination may have to wait due to manufacturing constraints, which could be exacerbated if multiple doses are required. Adar Poonawlaa, CEO of the Serum Institute of India (the world’s largest vaccine manufacturer) said it would take four to five years to vaccinate everyone on the planet.8 More disconcerting was a recent comment made by David Morens, virologist at the National Institute of Allergy and Infectious Diseases and senior advisor to Fauci, director of the NIAID: “Although we don’t know yet, it is well within the realm of possibility that this coronavirus, when our population-level immunity gets high enough, will find a way to get around our immunity. If that happened, we’d be in the same situation as with flu. We’ll have to chase the virus and, as it mutates, we’ll have to tinker with our vaccine.”9
References
- bit.ly/3e6M6rG
- bit.ly/3osKahV
- bit.ly/3oqVISR
- bit.ly/35Cee1L
- bit.ly/3mstR2y
- bit.ly/3jtOQ39
- bit.ly/31LshB1
- bit.ly/35DYsn1
- bit.ly/35DYBqz
Eric Williams leads Capstone’s Health and Medical practice. Previously, he managed the Eastern region for Morgan Stanley Smith Barney’s Capital Strategies Group and its predecessor, Citi Capital Strategies. Eric has more than 20 years of experience managing strategic sale assignments and leveraged recapitalizations for owners of privately held companies. He has successfully completed over 100 transactions in a various subsectors including medical device, diagnostics, contract manufacturing, and pharmaceutical outsourcing. He is a Series 7 and 63 Registered Securities Representative as well as a Series 24 Registered Securities Principal.