75-Minute Diagnostic Determines COVID Status
By Abacus Diagnostica | 09.24.20
Abacus Diagnostica is also developing a multiplex assay for COVID, influenza, and RSV.
Abacus Diagnostica is a small Finnish company developing and manufacturing assays and de-vices for rapid molecular diagnostics. Since the beginning of COVID-19 outbreak the company has worked decisively on the development of SARS-CoV-2 diagnostics. In July 2020, their SARS-CoV-2 assay for the GenomEra CDX system received the CE IVD approval for the European mar-ket. The results from the PCR-based COVID-19 test are available within 75 minutes. Because of the ease and rapidity of the system, many hospitals are using the assay for urgent samples such as testing the hospital personnel.
“Thanks to our committed and competent team, we succeeded to develop and launch the new COVID-19 assay within a few months, and then increased our production capacity to meet the massive demand. Despite the global crisis and scarcity of some supply materials, we have managed to keep our production schedules and the reliability of delivery,” said Erno Sundberg, CEO of Abacus Diagnostica.
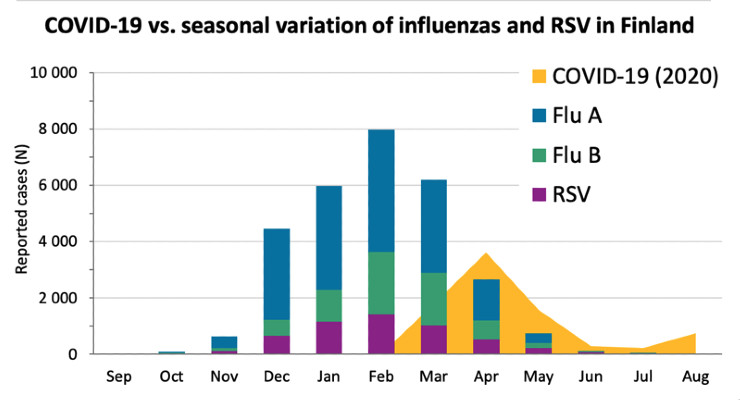
In addition to the COVID-19 assay, the company has been working on a four-in-one test kit for detecting influenza A, influenza B, RSV and SARS-CoV-2. The seasonal variation of both influ-enzas and RSV is evident (Table). Every year these common viruses cause humane, social and economic burden for the people and the communities. In the Northern hemisphere, the flu sea-son is approaching now on top of the COVID-19 pandemic. The health care system and clinical laboratories are already working on full capacity because of COVID-19, and that is a concern to Sundberg.
Table
“The COVID-19 testing is essential to manage and control the pandemic. When everyone is fo-cused on COVID-19, there is a risk of delays on the diagnosis and treatment of other infections. It would benefit the patients and save the resources of the health care system to test more than one pathogen at a time. The flu season awaits in the shade of COVID-19 outbreak, and our new assay will help to bring it on sight as soon as it sets off,” he explained.
Sundberg is confident with the company’s plans to seek the CE IVD approval for the multiplexed assay detecting influenzas, RSV and SARS-CoV-2. The time frame is set by the approaching flu season. Abacus Diagnostica is aiming for the market launch with well available, CE IVD ap-proved four-in-one assay in November, in a good time before the flu season begins.
“Thanks to our committed and competent team, we succeeded to develop and launch the new COVID-19 assay within a few months, and then increased our production capacity to meet the massive demand. Despite the global crisis and scarcity of some supply materials, we have managed to keep our production schedules and the reliability of delivery,” said Erno Sundberg, CEO of Abacus Diagnostica.
In addition to the COVID-19 assay, the company has been working on a four-in-one test kit for detecting influenza A, influenza B, RSV and SARS-CoV-2. The seasonal variation of both influ-enzas and RSV is evident (Table). Every year these common viruses cause humane, social and economic burden for the people and the communities. In the Northern hemisphere, the flu sea-son is approaching now on top of the COVID-19 pandemic. The health care system and clinical laboratories are already working on full capacity because of COVID-19, and that is a concern to Sundberg.

Table
“The COVID-19 testing is essential to manage and control the pandemic. When everyone is fo-cused on COVID-19, there is a risk of delays on the diagnosis and treatment of other infections. It would benefit the patients and save the resources of the health care system to test more than one pathogen at a time. The flu season awaits in the shade of COVID-19 outbreak, and our new assay will help to bring it on sight as soon as it sets off,” he explained.
Sundberg is confident with the company’s plans to seek the CE IVD approval for the multiplexed assay detecting influenzas, RSV and SARS-CoV-2. The time frame is set by the approaching flu season. Abacus Diagnostica is aiming for the market launch with well available, CE IVD ap-proved four-in-one assay in November, in a good time before the flu season begins.