By Sean Fenske, Editor-in-Chief
Molding is an instrumental process for the manufacture of critical components for medical devices. An overwhelming amount of healthcare products have plastic within them. Yet, the injection molding process has largely been performed as an artform rather than a scientific one. A number of companies have been working for years to change that.
There are a variety of technologies that can help put data and science into the molding process. These instruments help to ensure consistency, accuracy, reliability, and safety (for patients). It also helps provide real information for quality verification of finished goods. This information can be used for several purposes that all ultimately save a device maker time and money.
In the following Q&A, Marty Key, senior consultant and trainer at RJG Inc., has shared his expertise on adding instrumentation to the injection molding process. He speaks to the advantages realized, the challenges he’s encountered, and why this is important for medical device manufacturers.
Sean Fenske: How is injection molding traditionally configured/measured and how is that changing?
Marty Key: The traditional methods of injection molding are quickly becoming antiquated. Molding was once a process of heating plastic and injecting it at a certain amount of pressure for a certain period of time. Collecting data from the process was almost nonexistent. A few timers and limit switch locations documented in a notebook were once an industry standard.
Over the years, the industry has rapidly evolved. Machine manufacturers and third-party companies have rapidly advanced technology to begin capturing data from the process. The industry has evolved from a minimalist approach and no recorded process to an abundance of information on temperature, velocities, pressures, times, and integrals, to name a few. Molders can now use the instrumentation in the form of transducers, thermocouples, and encoders to gather an enormous amount of data on the molding process. This data can help molders monitor and control the process, reduce rejects, and formulate a preventative maintenance plan.
Fenske: What does the use of instrumentation in injection molding provide to the molder and OEM customer?
Key: First, let’s cover what instrumentation is. The use of instrumentation in injection molding refers to placing sensors throughout the molding process. For instance, reading temperature from a thermocouple is a form of instrumenting the process. Instrumentation has seen major advancements over the past few decades. Now literally every facet of the process can be monitored through instrumentation. The closer to the final product we can gather information, the more valuable the data we gather can become. Instrumentation has advanced to placing sensors inside of the mold to record information. Pressure transducers, thermocouples, and deflection sensors are all placed within the mold’s cavities and cores to gather information on the final product, while it is being manufactured.
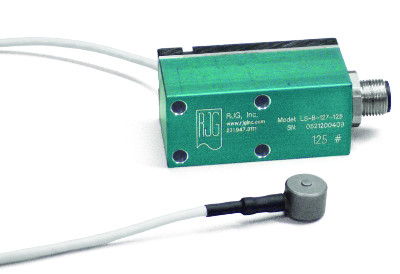
The in-mold transducer is used for recording pressure within the mold.
The machine gathers lots of data points but by instrumenting the mold, the manufacturer can see how machine inputs and outputs affect the final product. If we look at a common scenario in injection molding, the short-shot or non-fill is produced when a molded component is not filled during the process. This creates several issues.
The first is there is a defect that has been created. Without instrumentation, we wouldn’t know the defects have been created, nor would we know how many, or where there may be located within a batch or lot of parts. We are at the mercy of an operator or visual inspection from quality to catch and discard the component. This scenario is not only costly and time-consuming but risky at best. What if the parts are not found at all, or what if the sorting of the parts is not effective and we are still left with defective products in the lot. Those parts have the potential to wind up in finished goods or reach the customer. A very costly mistake.
With instrumentation, we can easily correct this scenario. By placing a pressure transducer within the mold at the last portion of the mold to fill, we can determine before the mold opens whether the component is a full, complete component or a short shot. A signal can be configured to reject the components in the mold. When set up correctly, the transducer data can reject all non-filled defective components—eliminating the costly, time-consuming, and often unreliable quality control method or sorting.
Besides knowing if a part is full or not, molders can also use the data they gather from the process to track and detect normal and abnormal process variations. Molders can now begin to understand and predict what will happen in the process as they see shifts in variation.
Fenske: Are manufacturers seeking more data about the injection molding process?
Key: Absolutely—with data comes a better understanding of the process and what affects it. Supplier and regulatory agencies are also pushing for more data-driven decisions. Using instrumentation and the data collected can enable floor-level employees through management to make informed decisions. For example, technicians and engineers are often in charge of developing and adjusting processes. Often without instrumentation and data, there is no real way to quantify how these adjustments affect the final product. With instrumentation and a visual graph representation of process parameters, like pressures and temperatures, the engineer and technician can see how changes affect not only the process but the final product. Technicians and engineers can now work with quality engineers and managers to determine what data is relevant to the process. All relevant data can now be continuously monitored and parameters can be placed on factors critical to quality. The more data collected from the process, the more confident the molder can be that not only have they created a robust process but they are sending quality components to their customer.
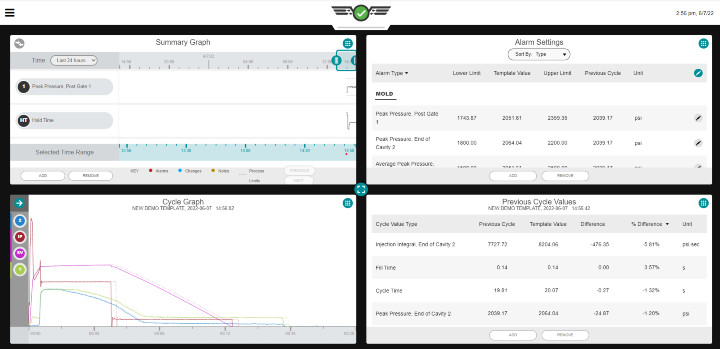
Photo to show cavity pressure curves and the data being collected.
Gathering data on the front end allows molders to build a consistent and repeatable process. More and more molders are using instrumentation while qualifying a mold, process, or manufacturing cell. The data collected during the process development, DOE (design of experiments), OQ (operational qualification), and PQ (performance qualification) phase can all be used to choose the right outputs to monitor and determine the limits of those outputs.
Fenske: Does access to data from the injection molding process and use of instrumentation have an impact on the output (or the ability to attain more consistent results)?
Key: Data and instrumentation can have a huge impact on the output of the molding process. We have already chronicled some of these advantages previously. Cavity pressure data from the mold can reject defects before the cycle is complete. If the process continues to yield bad products the system can be configured to shut the process down until the issue can be addressed and resolved. This again reduces the number of rejects being produced. The biggest advantage is the process itself is driven by data. For example, while the process is being developed, we can determine what process parameters affect critical quality criteria. Having a pressure transducer in the cavity can detect a non-fill; we know zero pressure equals a defect.
But we can take this approach a step further. If we can show a correlation between cavity pressure and quality criteria, we can begin to create a known process window. Let’s say we know zero pressure is a non-fill and 1,000 psi is a full shot, but dimensionally, the parts are below the low specification. When we hit 2,000 psi, our dimensions are within specification and remain in compliance until we reach 5,000 psi, when we know the dimension goes above the upper specification. At this point, we know our dimensional criteria are satisfied when the pressure transducer within the mold is 2,000 to 4,999 psi. We can now set limits within the data collection device that can continuously monitor this pressure. Once the pressure goes above or below the lower or upper limits, the system can automatically reject suspect components. The instrumentation and data derived from the instrumentation is giving the organization the ability to make an informed decision about where the process should run. This, in turn, creates a process that has the potential to run uninterrupted and produce a maximum output while automatically sorting defects.
Fenske: What are the challenges associated with using instrumentation in injection molding and how do you resolve them?
Key: The biggest challenge of instrumenting injection molding is educating the staff on the use and benefits of the technology. A commitment from management to create an implementation plan is a huge step to overcoming these challenges. The technology has major advantages and can transform not only processes but companies. However, there has to be a plan to introduce the technology, show the employees how they can benefit from the technology, then allow them to use the technology. If engineers and technicians can see the benefits of visualizing the process and changes made to the process, quality can see the enormous amounts of data collected from instrumenting the process.
Fenske: Have you found there are additional, unexpected benefits of using instrumentation in injection molding for medical devices?
Key: There are several other benefits to instrumenting a molding process. Tracking data over time can give the molder an idea of when maintenance needs to occur. Instead of waiting for components to break and producing scrap and downtime along the way, molders can now see data trends points to component wear.
Instrumentation also allows the molder to remotely monitor and control the process. Often, as issues arise, the needed personnel are out of the facility. Allowing remote access to the process can help return the process to production at a faster rate.
One of the biggest advantages of instrumenting a molding process for a medical device is the cost savings from validating and transferring a mold and process. Often molders spend a large amount of time validating a tool at one location to move the mold to another location just to repeat the long, costly validation process. By instrumenting the mold, we can record pressure and temperature curves from inside the mold itself. Knowing these curves correlate to critical quality criteria, we can create a baseline or template. Once the mold is transferred to a new location or supplier, the curves can then be matched against the template, drastically reducing the validation time and cost.
Fenske: Do you have any additional comments you’d like to share based on any of the topics we discussed or something you’d like to tell medical device manufacturers?
Key: Instrumentation can not only be used to monitor the injection molding process but control it. Molders constantly deal with shifts in plastic viscosity. This viscosity shift can result in various molding-related defects. When the mold is instrumented, the process control point is then located within the mold where the final product is produced. The transducer can signal the machine to transfer from velocity control to pressure control, thus limiting a majority of the variation in viscosity.
Click here to learn more about RJG >>>>>
Molding is an instrumental process for the manufacture of critical components for medical devices. An overwhelming amount of healthcare products have plastic within them. Yet, the injection molding process has largely been performed as an artform rather than a scientific one. A number of companies have been working for years to change that.
There are a variety of technologies that can help put data and science into the molding process. These instruments help to ensure consistency, accuracy, reliability, and safety (for patients). It also helps provide real information for quality verification of finished goods. This information can be used for several purposes that all ultimately save a device maker time and money.
In the following Q&A, Marty Key, senior consultant and trainer at RJG Inc., has shared his expertise on adding instrumentation to the injection molding process. He speaks to the advantages realized, the challenges he’s encountered, and why this is important for medical device manufacturers.
Sean Fenske: How is injection molding traditionally configured/measured and how is that changing?
Marty Key: The traditional methods of injection molding are quickly becoming antiquated. Molding was once a process of heating plastic and injecting it at a certain amount of pressure for a certain period of time. Collecting data from the process was almost nonexistent. A few timers and limit switch locations documented in a notebook were once an industry standard.
Over the years, the industry has rapidly evolved. Machine manufacturers and third-party companies have rapidly advanced technology to begin capturing data from the process. The industry has evolved from a minimalist approach and no recorded process to an abundance of information on temperature, velocities, pressures, times, and integrals, to name a few. Molders can now use the instrumentation in the form of transducers, thermocouples, and encoders to gather an enormous amount of data on the molding process. This data can help molders monitor and control the process, reduce rejects, and formulate a preventative maintenance plan.
Fenske: What does the use of instrumentation in injection molding provide to the molder and OEM customer?
Key: First, let’s cover what instrumentation is. The use of instrumentation in injection molding refers to placing sensors throughout the molding process. For instance, reading temperature from a thermocouple is a form of instrumenting the process. Instrumentation has seen major advancements over the past few decades. Now literally every facet of the process can be monitored through instrumentation. The closer to the final product we can gather information, the more valuable the data we gather can become. Instrumentation has advanced to placing sensors inside of the mold to record information. Pressure transducers, thermocouples, and deflection sensors are all placed within the mold’s cavities and cores to gather information on the final product, while it is being manufactured.

The in-mold transducer is used for recording pressure within the mold.
The first is there is a defect that has been created. Without instrumentation, we wouldn’t know the defects have been created, nor would we know how many, or where there may be located within a batch or lot of parts. We are at the mercy of an operator or visual inspection from quality to catch and discard the component. This scenario is not only costly and time-consuming but risky at best. What if the parts are not found at all, or what if the sorting of the parts is not effective and we are still left with defective products in the lot. Those parts have the potential to wind up in finished goods or reach the customer. A very costly mistake.
With instrumentation, we can easily correct this scenario. By placing a pressure transducer within the mold at the last portion of the mold to fill, we can determine before the mold opens whether the component is a full, complete component or a short shot. A signal can be configured to reject the components in the mold. When set up correctly, the transducer data can reject all non-filled defective components—eliminating the costly, time-consuming, and often unreliable quality control method or sorting.
Besides knowing if a part is full or not, molders can also use the data they gather from the process to track and detect normal and abnormal process variations. Molders can now begin to understand and predict what will happen in the process as they see shifts in variation.
Fenske: Are manufacturers seeking more data about the injection molding process?
Key: Absolutely—with data comes a better understanding of the process and what affects it. Supplier and regulatory agencies are also pushing for more data-driven decisions. Using instrumentation and the data collected can enable floor-level employees through management to make informed decisions. For example, technicians and engineers are often in charge of developing and adjusting processes. Often without instrumentation and data, there is no real way to quantify how these adjustments affect the final product. With instrumentation and a visual graph representation of process parameters, like pressures and temperatures, the engineer and technician can see how changes affect not only the process but the final product. Technicians and engineers can now work with quality engineers and managers to determine what data is relevant to the process. All relevant data can now be continuously monitored and parameters can be placed on factors critical to quality. The more data collected from the process, the more confident the molder can be that not only have they created a robust process but they are sending quality components to their customer.

Photo to show cavity pressure curves and the data being collected.
Gathering data on the front end allows molders to build a consistent and repeatable process. More and more molders are using instrumentation while qualifying a mold, process, or manufacturing cell. The data collected during the process development, DOE (design of experiments), OQ (operational qualification), and PQ (performance qualification) phase can all be used to choose the right outputs to monitor and determine the limits of those outputs.
Fenske: Does access to data from the injection molding process and use of instrumentation have an impact on the output (or the ability to attain more consistent results)?
Key: Data and instrumentation can have a huge impact on the output of the molding process. We have already chronicled some of these advantages previously. Cavity pressure data from the mold can reject defects before the cycle is complete. If the process continues to yield bad products the system can be configured to shut the process down until the issue can be addressed and resolved. This again reduces the number of rejects being produced. The biggest advantage is the process itself is driven by data. For example, while the process is being developed, we can determine what process parameters affect critical quality criteria. Having a pressure transducer in the cavity can detect a non-fill; we know zero pressure equals a defect.
But we can take this approach a step further. If we can show a correlation between cavity pressure and quality criteria, we can begin to create a known process window. Let’s say we know zero pressure is a non-fill and 1,000 psi is a full shot, but dimensionally, the parts are below the low specification. When we hit 2,000 psi, our dimensions are within specification and remain in compliance until we reach 5,000 psi, when we know the dimension goes above the upper specification. At this point, we know our dimensional criteria are satisfied when the pressure transducer within the mold is 2,000 to 4,999 psi. We can now set limits within the data collection device that can continuously monitor this pressure. Once the pressure goes above or below the lower or upper limits, the system can automatically reject suspect components. The instrumentation and data derived from the instrumentation is giving the organization the ability to make an informed decision about where the process should run. This, in turn, creates a process that has the potential to run uninterrupted and produce a maximum output while automatically sorting defects.
Fenske: What are the challenges associated with using instrumentation in injection molding and how do you resolve them?
Key: The biggest challenge of instrumenting injection molding is educating the staff on the use and benefits of the technology. A commitment from management to create an implementation plan is a huge step to overcoming these challenges. The technology has major advantages and can transform not only processes but companies. However, there has to be a plan to introduce the technology, show the employees how they can benefit from the technology, then allow them to use the technology. If engineers and technicians can see the benefits of visualizing the process and changes made to the process, quality can see the enormous amounts of data collected from instrumenting the process.
Fenske: Have you found there are additional, unexpected benefits of using instrumentation in injection molding for medical devices?
Key: There are several other benefits to instrumenting a molding process. Tracking data over time can give the molder an idea of when maintenance needs to occur. Instead of waiting for components to break and producing scrap and downtime along the way, molders can now see data trends points to component wear.
Instrumentation also allows the molder to remotely monitor and control the process. Often, as issues arise, the needed personnel are out of the facility. Allowing remote access to the process can help return the process to production at a faster rate.
One of the biggest advantages of instrumenting a molding process for a medical device is the cost savings from validating and transferring a mold and process. Often molders spend a large amount of time validating a tool at one location to move the mold to another location just to repeat the long, costly validation process. By instrumenting the mold, we can record pressure and temperature curves from inside the mold itself. Knowing these curves correlate to critical quality criteria, we can create a baseline or template. Once the mold is transferred to a new location or supplier, the curves can then be matched against the template, drastically reducing the validation time and cost.
Fenske: Do you have any additional comments you’d like to share based on any of the topics we discussed or something you’d like to tell medical device manufacturers?
Key: Instrumentation can not only be used to monitor the injection molding process but control it. Molders constantly deal with shifts in plastic viscosity. This viscosity shift can result in various molding-related defects. When the mold is instrumented, the process control point is then located within the mold where the final product is produced. The transducer can signal the machine to transfer from velocity control to pressure control, thus limiting a majority of the variation in viscosity.
Click here to learn more about RJG >>>>>













