Outsourcing Directory
Company Headquarters
125 Highpower Road
Rochester, NY 14623
United States
Company Description
We have extensive regulatory experience with the FDA and regulatory bodies worldwide. Test protocols and final reports follow the most current national or international standards, are tailored to each device, and have a long history of acceptance by the FDA during a 510(k) review. HIGHPOWER Labs is an ISO 17025 accredited laboratory and is ASP factory trained to perform STERRAD® functionality and efficacy validation testing. We provide a wide range of verification, validation and testing services, as well as consulting services to medical device manufacturers.
Outsourcing Directory
In-Vitro Diagnostic Categories
Related Content
-

ASTM International Group Approves Guide for Cleaning Reusable Medical Devices
The guide is an important step in the process of validating cleaning instructions that healthcare workers perform to prepare a device for the next patient.ASTM International 11.20.19
-
Cardiovascular | Diagnostics

-
Diagnostics

CareDx’s KidneyCare iBox Technology Clinically Validated in BMJ Publication
Artificial intelligence algorithm predicts long-term kidney transplant outcomes.CareDx Inc. 09.25.19
-
Materials
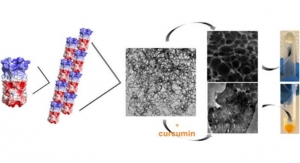
Researchers Develop Hydrogel Capable of Providing Sustained Medication Release
Delivers drugs at the cellular level and engineers tissue.NYU Tandon 09.18.19
-
Packaging & Sterilization

Revisiting the End: A Packaging and Sterilization Update
Changing regulations and new demands from medical device manufacturers are causing packaging and sterilization suppliers to revise and update their offerings.Mark Crawford, Contributing Writer 06.13.19
-
Packaging & Sterilization
Package Testing and Validation: Interplay Between ISO 11607 Updates and MDR
...Britt Jones, Manager of Package Testing Services, WuXi AppTec 06.13.19
-












