YOU SEARCHED BY...
SEARCH BY...
Outsourcing Directory
Country
- Canada(2)
- China(2)
- Costa Rica(1)
- France(2)
- Germany(2)
- India(3)
- Italia (2)
- Japan(1)
- Lithuania(1)
- Mexico(1)
- New Zealand(1)
- Singapore(3)
- Switzerland(3)
- Taiwan(4)
- The Dominican Republic(1)
- United Kingdom(3)
- United States(342)
Outsourcing Directory
Company Headquarters
1295 Polk Drive
Warsaw, IN 46580
United States
Company Description
Edge International, based in Dayton, Ohio is an ISO 13485 distributor of medical grade Cobalt & Stainless Bar and Titanium Bar, Plate & Sheet for the manufacture of implants & instruments for the orthopedic, spine & trauma markets. We work with customers to provide cost-effective solutions with the highest level of compliance, quality and service.
Outsourcing Directory
Medical Device Categories
Related Content
-
Diagnostics

Want to Improve Dipstick Diagnostic Tests? Just Add Tape
Researchers have developed a way to make these low-cost devices more versatile and reliable.American Chemical Society 11.30.17
-
Cardiovascular

-
Electronics | Neurological | Patient Monitoring
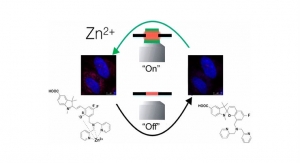
Australian Researchers Develop New Zinc Sensor
Excess zinc in the body is a possible sign of Alzheimer's or Parkinson's disease.University of Adelaide 10.02.17
-
3D/Additive Manufacturing | Materials

3D Metal Printing Opportunities in Medical Device Manufacturing
Technology contributes to the ushering in of personalized medicine in healthcare.Ed Potoczak, Director of Industry Relations, IQMS 09.01.17
-

Qosina Acquires Alpha Industries Inc.
Disposable component manufacturer’s full product line now available on Qosina’s website.Qosina Corporation 07.25.17
-

Qosina Acquires Alpha Industries, Inc.
A disposable component manufacturer headquartered in Clearwater, FL.
-
Diagnostics

Applied BioCode Achieves ISO 13485:2003 Certification
Authorization received for the design, development, manufacture and distribution of in-vitro diagnostic reagents.Business Wire 06.12.17












