YOU SEARCHED BY...
SEARCH BY...
Outsourcing Directory
Country
- Canada(2)
- China(2)
- Costa Rica(1)
- France(2)
- Germany(2)
- India(3)
- Italia (2)
- Japan(1)
- Lithuania(1)
- Mexico(1)
- New Zealand(1)
- Singapore(3)
- Switzerland(3)
- Taiwan(4)
- The Dominican Republic(1)
- United Kingdom(3)
- United States(342)
Outsourcing Directory
Company Headquarters
101 Columbus Street
Auburn, NY 13021
United States
Company Description
Custom Molding Solutions. Injection & Blow Molding in a highly competitive environment, design innovation combined with the right costs and precise time-to-market manufacturing can make the difference in improving your market sales opportunities and product performance. To speak to a Business Development Manager, email us at Info@CurrierPlastics.com.
Outsourcing Directory
In-Vitro Diagnostic Categories
Related Content
-

United Biologics Appoints President and General Manager
New hire has brings over 20 years of experience in commercial leadership, primarily in the medical device industry.Business Wire 11.07.19
-
Contract Manufacturing

Sales Tips for Contract Manufacturers of Finished Devices
Evolving trends in the supply chain may require companies to consider a strategy of selling devices of their own design.Tony Freeman, President, A.S. Freeman Advisors LLC 11.04.19
-

Attorney General Morrisey Sues Johnson & Johnson
Alleges that the company engaged in unlawful and deceptive marketing practices.West Virginia Attorney General's Office 09.19.19
-
Cardiovascular | Contract Manufacturing | Diagnostics | Digital Health | Electronics | Neurological | Patient Monitoring | R&D & Design

Customized Connections: A Discussion on Custom Electronics
Demand for more complex, traceable, integrated solutions are driving innovation in the fast-changing medical electronics market.Michael Barbella, Managing Editor 09.09.19
-
Cardiovascular
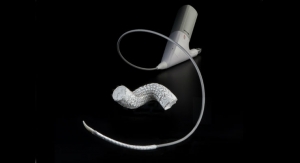
First U.S. Patient Receives GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL
Data shows 100 percent successful deployment and no fractures at 30-day follow-up in European Registry.Business Wire 08.30.19
-

Boyd Corporation Announces ISO 13485:2016 Certification of Southern Asia Facility
Company expands its medical Quality Management Systems for medical devices in South Asia.Business Wire 08.29.19
-

Motus GI Adds Two Key Senior Managers to its Executive Team
Management team to drive commercial launch of Pure-Vu GEN2 in the U.S. hospital market.Business Wire 08.06.19
-
Cardiovascular
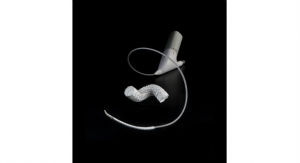
FDA Approves the GORE TAG Conformable Thoracic Stent Graft With ACTIVE CONTROL
The device and new delivery system optimize TEVAR outcomes in complex aortic anatomy.Business Wire 07.01.19
-
Contract Manufacturing

Tariffs and Nearshoring with Providien at MD&M East
Ryan McCormick of Providien talks about tariffs affecting the industry and the efforts the company is making to address them.Sam Brusco, Associate Editor
-

Currier Plastics Appoints a Director of Manufacturing and Project Manager
New executive currently chairs the Injection Molding Division Board of Directors of Society of Plastics Engineers (SPE).Currier Plastics Inc. 10.20.17
-
Materials
Currier Plastics Hires New VP of Business Development
Ron Ringleben most recently spent 16 years at Thermo Fisher Scientific.Currier Plastics 09.06.16












