YOU SEARCHED BY...
SEARCH BY...
Outsourcing Directory
Country
- Canada(2)
- China(2)
- Costa Rica(1)
- France(2)
- Germany(2)
- India(3)
- Italia (2)
- Japan(1)
- Lithuania(1)
- Mexico(1)
- New Zealand(1)
- Singapore(3)
- Switzerland(3)
- Taiwan(4)
- The Dominican Republic(1)
- United Kingdom(3)
- United States(342)
Outsourcing Directory
Creganna Medical, part of TE Connectivity
Company Headquarters
Parkmore West
Galway, H91VN2T
Ireland
Outsourcing Directory
More Content From Creganna Medical, part of TE Connectivity
-
Creganna-Tactx Medical live from PCR
Live from PCR, we present the contract design service for MI medical devices at Creganna-Tactx Medical. Discussion of our customer challenges in product desing including some case examples and recent milestones achieved.Videos Released on 03.11.2019
-
Braiding at Creganna-Tactx Medical presented by Jeff Kraus
In this video, Jeff Kraus, Head of Engineering at Creganna-Tactx Medical & a braid development specialist provides an overview of the company's braiding capabilities worldwide. Jeff presents the unique design challenges for braided shafts in neurovascular catheter and deflecting catheter applications.Videos Released on 03.11.2019
-
Braided Shafts at Creganna-Tactx Medical
Overview of braided shaft capability at Creganna-Tactx Medical. Including delivery catheters, steerable catheters, lead placement devices and microcatheters. Unique braid termination technology.Videos Released on 03.11.2019
-
Creganna-Tactx Medical, Introduction
Introduction to Creganna-Tactx MedicalVideos Released on 03.11.2019
-
Manufacturing Transfers
Creganna-Tactx Medical has a proven track record in transferring customer’s existing steady-state device manufacturing into its facilities.Services Released on 03.11.2019
-
Design Services
Creganna-Tactx Medical’s contract design and development service enables you to bring products to market quickly and efficiently.Services Released on 03.11.2019
-
Your Service Network
Creganna Medical’s end-to-end solution set and quality record deliver maximum efficiency over the lifecycle of your product.Literature / Brochures Released on 03.11.2019
-
Laser Engineering Reference Guide
TE Connectivity (TE) expanded its laser capabilities through the acquisition of LSA Laser.Literature / Brochures Released on 03.11.2019
-
The Secret of SmartForm
Creganna Medical is the only outsourcing partner with both FDA registration and balloon capability across three continents.Literature / Brochures Released on 03.11.2019
-
Minimally Invasive Device Segments from Creganna Medical
Read about a variety of Minimally Invasive Device Solutions from Creganna MedicalLiterature / Brochures Released on 03.11.2019
-
Major Expansion for Creganna Medical, Minnesota
Creganna Medical opens new manufacturing facility in Plymouth, MinnesotaPress Releases Released on 11.18.2015
-
Creganna-Tactx Medical to Acquire Precision Wire Components
Strengthens Position as a Global Leader in Medical TechnologiesPress Releases Released on 10.29.2014
Related Content
-

Catheter and Medical Design Hires Two Industry Experts
Rich Abraham and Scott Olson will lead business development and customer relationships in the East Coast and Midwest markets.Catheter and Medical Design LLC 07.16.21
-

Wytech Industries Appoints President
David Ohmann brings 25 years of experience in international, orthopedic, and advanced surgical medical device segments.Charles Sternberg, Assistant Editor 05.05.21
-
Cardiovascular | Neurological

Imperative Care Closes a Series C Financing of $85 Million for Stroke Treatments
The funds will support the commercial launch of the company’s portfolio.Imperative Care Inc. 12.10.19
-
Tubing & Extrusion

-
Software & IT

Survey Identifies Challenges to Improving Product Development Processes
81% of medical device companies are not using tools designed for the medical device industry in their quality management processes.PRWeb 11.26.19
-
Materials
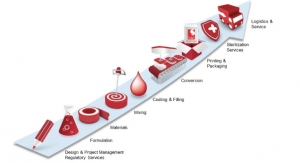
5 Questions for Scapa Healthcare at Medica/CompaMed 2019
Formulation development, adhesive coating, filling, converting, printing, and packaging for skin-contact adhesive and skin care topical solutions.Sean Fenske, Editor-in-Chief 11.15.19
-
Packaging & Sterilization

Anxious Undertones: The 2019 Year in Review
Despite a healthy U.S. economy and solid corporate growth, the medtech industry was wracked with worries in 2019.Michael Barbella, Managing Editor 11.04.19
-

Freudenberg Expands Medical Balloon Development
Establishes the Balloon Development Center of Excellence.Freudenberg Medical 11.04.19
-
Diagnostics

Beckman Coulter Receives BARDA Funding
Is developing an algorithm to aid in the early detection of sepsis.Beckman Coulter 10.30.19
-
Software & IT

HSCC Publishes Guidance on Cybersecurity Risk Management
Provides actionable guidance and practical tools.HSCC 10.16.19
-
Cardiovascular | Surgical

Edwards Invests $100M to Expand Operations in Costa Rica
The company expects to have 1,100 employees by the end of 2020 in its facility at La Lima Industrial Park in Cartago.PR Newswire 10.11.19
-
Cardiovascular | Surgical

Medtronic Recalls Catheters Due to Separation and Fragmentation Issue
Medtronic is recalling the 6 French Sherpa NX Active Guide Catheter due to a risk of the outer material separating from the device.U.S. Food and Drug Administration 10.08.19
-
Materials
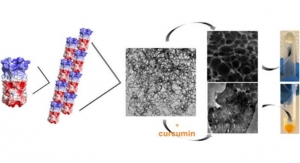
Researchers Develop Hydrogel Capable of Providing Sustained Medication Release
Delivers drugs at the cellular level and engineers tissue.NYU Tandon 09.18.19
-
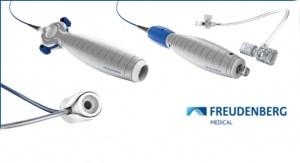
Freudenberg Medical Presents New Catheters and Hemostasis Valve
Reveals new options for medical companies looking to accelerate time to market.Freudenberg Medical 09.18.19
-
Materials

Cortland Biomedical Opens State-of-the-Art Facility
Strategically designed by textile engineers.Cortland Biomedical 09.17.19
-
R&D & Design
Three Crucial Elements of Conducting International Usability Research
It is important to conduct usability research throughout the product development cycle.Joe Pratt, CHFP, Lead Human Factors Engineer, BlackHägen Design 09.06.19
-
Software & IT
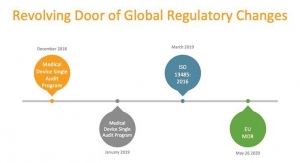
Five Strategies to Navigate Regulatory Challenges in Medical Device Development
...Seth J. Goldenberg, Ph.D., Vice President, Vault Medical Device & Diagnostics, Veeva Systems 07.30.19
-
Three Key Takeaways from Four International Medtech Clusters
...Mihir Torsekar, Analyst, U.S. International Trade Commission 07.30.19











