Cardiac Dimensions Enrolls First Patients in REDUCE FMR Clinical Trial
Cardiac Dimensions Enrolls First Patients in REDUCE FMR Clinical Trial
Study is evaluating a mitral valve repair device in patients with functional mitral regurgitation.
Randomizing 120 patients in 20 European and Australian hospitals, REDUCE FMR is intended to help establish Carillon as the "gold standard" treatment for functional mitral regurgitation (FMR), a condition in which blood flow is restricted from an abnormally enlarged mitral valve. All patients enrolled in the study are on an optimized heart failure medication regimen and then are randomized into two groups: one additionally treated with the Carillon device and the second remaining on an optimized regimen of heart failure medications, the current gold standard.
The study design contains several elements aimed at optimizing recruitment and enrollment, including a 3:1 randomization ratio allowing for more data to be collected with the Carillon device and a cross-over registry, which allows control patients to receive Carillon treatment at the end of their 12-month follow up. A built-in exercise echocardiographic sub-study will further evaluate the Carillon product’s ability to reduce mitral regurgitation, improve functional capacity and quality of life as well as induce reverse ventricular remodeling in a symptomatic heart failure patient population both at rest and during exercise.
The REDUCE FMR clinical trial follows three successful multi-center studies, featuring the Carillon device–-the AMADEUS, TITAN and TITAN II trials, according to the company.
“My experiences with Carillon have been extremely positive. As an investigator in the last two multi-center trials involving Carillon therapy I’ve seen significant clinical improvement in patients who receive the device,” said Janusz Lipiecki, M.D., of Clinique Pôle Santé République in Clermont Ferrand, France. “In this latest trial, we expect to firmly establish the magnitude of benefit that patients receive from Carillon.”
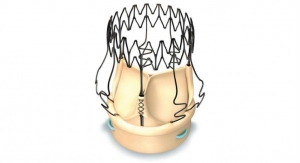
The Carillon Mitral Contour System is a percutaneous mitral annuloplasty treatment option that can be deployed rapidly and safely1 utilizing standard interventional techniques. The implantable device consists of a distal anchor and a proximal anchor connected by a shaping ribbon and uses the heart’s venous anatomy to reshape the mitral annulus. This approach allows for reduction of the dilated annulus, addressing a root cause of functional mitral regurgitation (FMR). Carillon has demonstrated compelling efficacy, significantly improving patients’ symptoms, mitral regurgitation and quality of life, Cardiac Dimensions executives said. In addition, all adjunctive treatment options remain available after using Carillon, making it an ideal first-line therapy for FMR, the company claims.
“The outcomes from REDUCE FMR will be added to the data already collected from nearly 100 treated patients evaluated during the three previous prospective trials,” said Rick Stewart, CEO of Cardiac Dimensions. “This is the first randomized, blinded study in the field of FMR, making its results of critical importance in understanding the clinical significance of the Carillon device in this patient population.”
An estimated 70 percent of the 26 million people worldwide with heart failure suffer from functional mitral regurgitation (FMR), industry statistics show. FMR typically results from the dilation of the left ventricle, which is the main pumping chamber in the heart. As the left ventricle increases in size, the mitral valve also expands. This dimensional increase leads to mitral regurgitation, which significantly reduces the amount of blood flow out of the left ventricle and on to the body and its organs. FMR has been associated with high rates of mortality, reduced functional capacity, poor quality of life and an increase in patient hospitalizations. Current mainstream therapies to address FMR are limited. A majority of patients become refractory to medical therapy, which is the current standard of care and traditional surgical intervention is associated with high rates of operative morbidity and mortality.
Cardiac Dimensions develops minimally invasive treatments for heart failure and related cardiovascular conditions. The company has operations in Kirkland, Wash.; Sydney, Australia and Offenbach, Germany.Watch the video below for an animation of the Carillon implant procedure: