PR Newswire12.06.17
Hologic Inc. announced that it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its Panther Fusion AdV/hMPV/RV assay, a multiplexed assay that runs on the new Panther Fusion system.
The new assay detects Adenovirus, human Metapneumovirus, and Rhinovirus. It is the third diagnostic assay available on the Panther Fusion system, complementing the Panther Fusion Flu A/B/RSV assay and the Panther Fusion Paraflu assay, which both received clearance in October 2017.
"Clearance and launch of the new Fusion AdV/hMPV/RV assay completes our initial set of modular assays for respiratory viruses," said Tom West, president of the Diagnostic Solutions division at Hologic. "We now offer a suite of molecular assays that help labs maximize their efficiency when running respiratory tests, in addition to the benefits of doing so on the fully automated Panther Fusion system."
A number of respiratory panels currently on the market require testing for 20 or more targets even when a physician has only requested three or four, making testing time-consuming and expensive for laboratories. The Panther Fusion assays offer a modular approach to syndromic respiratory testing via the ability to run one, two or all three assays from a single patient specimen.
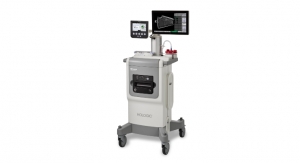
The Panther Fusion is available as a full system, or the Panther Fusion module can be attached to existing Panther systems in the field to extend testing capabilities. Specifically, the Panther Fusion module adds the capacity to run PCR (polymerase chain reaction) assays in addition to tests based on TMA (transcription-mediated amplification), the proprietary Hologic chemistry that powers the Company's Aptima brand. The Panther Fusion system retains all the key benefits of the Panther platform, including full sample-to-result automation, the ability to run multiple tests from a single sample, random and continuous access, sample processing with rapid turnaround time, continuous loading, and STAT capabilities.
Adenoviruses cause respiratory illnesses ranging from the common cold to pneumonia, croup, and bronchitis, and also can cause illnesses such as gastroenteritis, conjunctivitis, cystitis, and neurological disease.1 Infants and people with weakened immune systems are at high risk for developing severe illnesses caused by Adenovirus infection.1 hMPV is a common respiratory pathogen, particularly in infants and young children. The virus is associated with both upper and lower respiratory tract infections and may be a trigger for asthma.2 Clinical symptoms of hMPV infection may progress to bronchiolitis or pneumonia. Rhinoviruses are the causative pathogens in more than half of viral respiratory infections, and they are associated with acute exacerbations of respiratory disease, including asthma, sinusitis, otitis media, and COPD.3 A number of studies have confirmed rhinoviruses as being the most common cause of "the common cold."4
References
1https://www.cdc.gov/adenovirus/hcp/clinical-overview.html. Accessed December 1, 2017.
2Kahn, J.S., Epidemiology of human metapneumovirus. Clin Microbiol Rev, 2006. 19(3): p. 546-57.
3Anzueto, A. and M.S. Niederman. 2003. Diagnosis and treatment of rhinovirus respiratory infections. Chest 123:1664-1672.
4Park, J. Y., Yun, K. W., Lim, J. W., Lee, M. K., Lim, I. S., and Choi, E. S. (2016) Clinical and genetic features of human metapneumovirus infection in children. Pediatrics International, 58: 22–26. doi: 10.1111/ped.12782.
The new assay detects Adenovirus, human Metapneumovirus, and Rhinovirus. It is the third diagnostic assay available on the Panther Fusion system, complementing the Panther Fusion Flu A/B/RSV assay and the Panther Fusion Paraflu assay, which both received clearance in October 2017.
"Clearance and launch of the new Fusion AdV/hMPV/RV assay completes our initial set of modular assays for respiratory viruses," said Tom West, president of the Diagnostic Solutions division at Hologic. "We now offer a suite of molecular assays that help labs maximize their efficiency when running respiratory tests, in addition to the benefits of doing so on the fully automated Panther Fusion system."
A number of respiratory panels currently on the market require testing for 20 or more targets even when a physician has only requested three or four, making testing time-consuming and expensive for laboratories. The Panther Fusion assays offer a modular approach to syndromic respiratory testing via the ability to run one, two or all three assays from a single patient specimen.
The Panther Fusion is available as a full system, or the Panther Fusion module can be attached to existing Panther systems in the field to extend testing capabilities. Specifically, the Panther Fusion module adds the capacity to run PCR (polymerase chain reaction) assays in addition to tests based on TMA (transcription-mediated amplification), the proprietary Hologic chemistry that powers the Company's Aptima brand. The Panther Fusion system retains all the key benefits of the Panther platform, including full sample-to-result automation, the ability to run multiple tests from a single sample, random and continuous access, sample processing with rapid turnaround time, continuous loading, and STAT capabilities.
Adenoviruses cause respiratory illnesses ranging from the common cold to pneumonia, croup, and bronchitis, and also can cause illnesses such as gastroenteritis, conjunctivitis, cystitis, and neurological disease.1 Infants and people with weakened immune systems are at high risk for developing severe illnesses caused by Adenovirus infection.1 hMPV is a common respiratory pathogen, particularly in infants and young children. The virus is associated with both upper and lower respiratory tract infections and may be a trigger for asthma.2 Clinical symptoms of hMPV infection may progress to bronchiolitis or pneumonia. Rhinoviruses are the causative pathogens in more than half of viral respiratory infections, and they are associated with acute exacerbations of respiratory disease, including asthma, sinusitis, otitis media, and COPD.3 A number of studies have confirmed rhinoviruses as being the most common cause of "the common cold."4
References
1https://www.cdc.gov/adenovirus/hcp/clinical-overview.html. Accessed December 1, 2017.
2Kahn, J.S., Epidemiology of human metapneumovirus. Clin Microbiol Rev, 2006. 19(3): p. 546-57.
3Anzueto, A. and M.S. Niederman. 2003. Diagnosis and treatment of rhinovirus respiratory infections. Chest 123:1664-1672.
4Park, J. Y., Yun, K. W., Lim, J. W., Lee, M. K., Lim, I. S., and Choi, E. S. (2016) Clinical and genetic features of human metapneumovirus infection in children. Pediatrics International, 58: 22–26. doi: 10.1111/ped.12782.