Business Wire12.05.17
Toyoda Gosei Co. Ltd. has forged an agreement with EBM Corporation, a medical device startup at Waseda University in Japan, to develop and market surgical training simulators using e-Rubber, an advanced material Toyoda Gosei is currently developing.
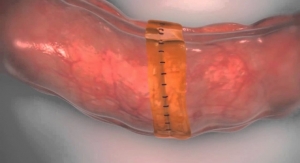
As surgeries become more advanced, ensuring safety is becoming increasingly important. Surgical training simulators that accurately reproduce the feel and movement of human organs are needed to improve surgical skills, especially for young doctors. Toyoda Gosei decided to work with EBM in order to achieve more life-like simulators by combining Toyoda Gosei’s e-Rubber technology, which takes advantage of the company’s expertise in polymers, and EBM’s accumulated achievements in developing surgical training simulators.
Toyoda Gosei will work with EBM in all areas, including product development and marketing, to explore the possibilities of e-Rubber for various purposes and contribute to the advancement of medical technology. e-Rubber is a versatile next-generation smart polymer that uses electromechanical transducers to transduce electric energy to or from mechanical movement, according to Toyoda Gosei.
Established in 2006 in Ota-ku, Tokyo, EBM is a university-launched venture business involved in training simulator development and system creation for both domestic Japanese and overseas markets, principally in the field of cardiac surgery. Two of its main products, the BEAT and YOUCAN coronary bypass surgical training simulators, are used in about 70 percent of cardiovascular surgery in Japanese hospitals. These simulators are also sold in the United States, Europe, and Asian countries. With the aim of international standardization of surgical technique training, EMS develops products based on a system of close cooperation with doctors from the aspects of both equipment and practices, centered on their “FIST” surgical training center in Fukushima City, Fukushima Prefecture, Japan.
Toyoda Gosei’s development of e-Rubber builds on the results of contract work in a New Energy and Industrial Technology Development Organization (NEDO) program for development of next-generation artificial intelligence and robot core technology. Findings obtained in the NEDO work are the basis for Toyoda Gosei’s development of new devices for next-generation robots, industrial equipment, automobiles, IoT and other areas to support aging societies.
Established in 1949 and headquartered in Kiyosu, Aichi Prefecture, Japan, Toyoda Gosei is a specialty manufacturer of rubber and plastic automotive parts and LEDs. The Toyoda Gosei Group provides a variety of products internationally, with a network of approximately 100 plants and offices in 18 countries and regions. Through its flexible, integrated global supply system and leading-edge technologies for automotive safety, comfort, and environmental preservation, Toyoda Gosei is a global supplier that aims to deliver the highest levels of quality, innovation, and satisfaction to customers worldwide.
As surgeries become more advanced, ensuring safety is becoming increasingly important. Surgical training simulators that accurately reproduce the feel and movement of human organs are needed to improve surgical skills, especially for young doctors. Toyoda Gosei decided to work with EBM in order to achieve more life-like simulators by combining Toyoda Gosei’s e-Rubber technology, which takes advantage of the company’s expertise in polymers, and EBM’s accumulated achievements in developing surgical training simulators.
Toyoda Gosei will work with EBM in all areas, including product development and marketing, to explore the possibilities of e-Rubber for various purposes and contribute to the advancement of medical technology. e-Rubber is a versatile next-generation smart polymer that uses electromechanical transducers to transduce electric energy to or from mechanical movement, according to Toyoda Gosei.
Established in 2006 in Ota-ku, Tokyo, EBM is a university-launched venture business involved in training simulator development and system creation for both domestic Japanese and overseas markets, principally in the field of cardiac surgery. Two of its main products, the BEAT and YOUCAN coronary bypass surgical training simulators, are used in about 70 percent of cardiovascular surgery in Japanese hospitals. These simulators are also sold in the United States, Europe, and Asian countries. With the aim of international standardization of surgical technique training, EMS develops products based on a system of close cooperation with doctors from the aspects of both equipment and practices, centered on their “FIST” surgical training center in Fukushima City, Fukushima Prefecture, Japan.
Toyoda Gosei’s development of e-Rubber builds on the results of contract work in a New Energy and Industrial Technology Development Organization (NEDO) program for development of next-generation artificial intelligence and robot core technology. Findings obtained in the NEDO work are the basis for Toyoda Gosei’s development of new devices for next-generation robots, industrial equipment, automobiles, IoT and other areas to support aging societies.
Established in 1949 and headquartered in Kiyosu, Aichi Prefecture, Japan, Toyoda Gosei is a specialty manufacturer of rubber and plastic automotive parts and LEDs. The Toyoda Gosei Group provides a variety of products internationally, with a network of approximately 100 plants and offices in 18 countries and regions. Through its flexible, integrated global supply system and leading-edge technologies for automotive safety, comfort, and environmental preservation, Toyoda Gosei is a global supplier that aims to deliver the highest levels of quality, innovation, and satisfaction to customers worldwide.