Business Wire04.19.17
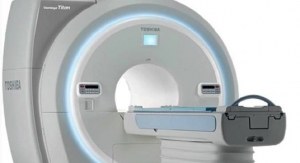
Healthcare providers can now offer enhanced care and safe imaging to patients with a compact and economical solution thanks to Toshiba Medical System Inc.'s Aquilion Lightning 80 CT system. The 80-detector row (160 slice) system is designed for full body imaging and routine volumetric scanning with the premium computed tomography (CT) technology found on high-end systems so that providers can deliver a better patient experience.
The Aquilion Lightning 80 is designed to be a total cost of ownership solution for the mid-tier CT space. The system delivers fast reconstruction speeds of up to 50 images per second at full resolution, and with the industry’s thinnest slices at 0.5 mm and a 78-cm bore, it optimizes workflow and patient comfort. To simplify exams, the Aquilion Lightning 80 comes standard with Adaptive Diagnostic solutions, such as Single Energy Metal Artifact Reduction (SEMAR) and SURESubtraction. The system can also be reconfigured from 40 to 80 to 160 slices without replacing hardware.
Design features of Aquilion Lightning 80 make this scanner a wide, fast and small solution for CT providers.
“The Aquilion Lightning 80 is designed to operate reliably and efficiently, producing high-quality images that can potentially help with faster diagnoses and treatment in a busy environment,” said Dominic Smith, senior director of the CT, PET/CT, and MR Business Units at Toshiba America Medical Systems Inc. “Additionally, providers don’t have to choose between a cost-effective solution and premium CT technology, as the Aquilion Lightning 80 features AIDR 3D Enhanced to help reduce dose and improve patient safety as well as the PUREViSION CT Detector.”
Toshiba America Medical Systems, a Canon Group company headquartered in Tustin, Calif., markets, sells, distributes and services radiology and cardiovascular systems, including CT, MR, ultrasound, X-ray and interventional X-ray equipment.
Toshiba Medical offers a full range of diagnostic medical imaging solutions including ultrasound, CT, X-Ray, and MR, across the globe. As of December 2016, Toshiba Medical became a member of the Canon Group.
The Aquilion Lightning 80 is designed to be a total cost of ownership solution for the mid-tier CT space. The system delivers fast reconstruction speeds of up to 50 images per second at full resolution, and with the industry’s thinnest slices at 0.5 mm and a 78-cm bore, it optimizes workflow and patient comfort. To simplify exams, the Aquilion Lightning 80 comes standard with Adaptive Diagnostic solutions, such as Single Energy Metal Artifact Reduction (SEMAR) and SURESubtraction. The system can also be reconfigured from 40 to 80 to 160 slices without replacing hardware.
Design features of Aquilion Lightning 80 make this scanner a wide, fast and small solution for CT providers.
“The Aquilion Lightning 80 is designed to operate reliably and efficiently, producing high-quality images that can potentially help with faster diagnoses and treatment in a busy environment,” said Dominic Smith, senior director of the CT, PET/CT, and MR Business Units at Toshiba America Medical Systems Inc. “Additionally, providers don’t have to choose between a cost-effective solution and premium CT technology, as the Aquilion Lightning 80 features AIDR 3D Enhanced to help reduce dose and improve patient safety as well as the PUREViSION CT Detector.”
Toshiba America Medical Systems, a Canon Group company headquartered in Tustin, Calif., markets, sells, distributes and services radiology and cardiovascular systems, including CT, MR, ultrasound, X-ray and interventional X-ray equipment.
Toshiba Medical offers a full range of diagnostic medical imaging solutions including ultrasound, CT, X-Ray, and MR, across the globe. As of December 2016, Toshiba Medical became a member of the Canon Group.