08.06.14
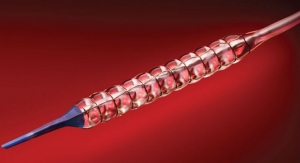
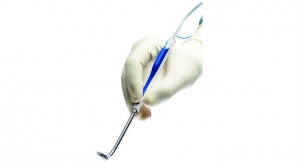
Minneapolis, Minn.-based Medtronic Inc. has launched its new product the Nuvent EM sinus dilation system for its Fusion ENT navigation system. Nuvent was developed by the medtech giant’s Ear Nose and Throat (ENT) division of its Surgical Technologies business.
According to Medtronic, Nuvent is the first and only balloon sinus dilation system with built-in electromagnetic (EM) surgical navigation technology to help the surgeon confirm anatomy and optimize balloon placement during balloon sinus surgery. The Nuvent system does not require complex set, and can simply be plugged in and used immediately. It provides surgeons with a view of the precise location of the Nuvent instrument’s tip on the Fusion screen. Balloon sinus surgery may be used as part of the treatment of chronic sinusitis, a disease that affects nearly 29 million U.S. adults.
When medical management fails to relieve symptoms, sinus surgery is often employed to restore normal mucociliary clearance in the sinuses. Surgical navigation is used in certain sinus procedures because sinus anatomy can be highly variable. The combination of the Nuvent system and Fusion ENT navigation provide an additional tool to help surgeons move tissue and bone around the openings of the frontal, maxillary, and sphenoid sinuses to help restore drainage in a minimally invasive way. In addition Medtronic is planning clinical trials to expand the indications for use of the Nuvent product to additional procedures, such as revision surgery.
“Nuvent is very appealing to customers because of its novel built-in surgical EM navigation, ease of use, and the familiar sinus instrument styles,” said Vince Racano, vice president and general manager of the ENT division at Medtronic. “This system is another example of our innovative integration of technologies and therapies that brings ‘Medtronic Surgical Synergy’ to the operating room, driving procedural excellence, economic value, and patient care.”
According to Medtronic, Nuvent is the first and only balloon sinus dilation system with built-in electromagnetic (EM) surgical navigation technology to help the surgeon confirm anatomy and optimize balloon placement during balloon sinus surgery. The Nuvent system does not require complex set, and can simply be plugged in and used immediately. It provides surgeons with a view of the precise location of the Nuvent instrument’s tip on the Fusion screen. Balloon sinus surgery may be used as part of the treatment of chronic sinusitis, a disease that affects nearly 29 million U.S. adults.
When medical management fails to relieve symptoms, sinus surgery is often employed to restore normal mucociliary clearance in the sinuses. Surgical navigation is used in certain sinus procedures because sinus anatomy can be highly variable. The combination of the Nuvent system and Fusion ENT navigation provide an additional tool to help surgeons move tissue and bone around the openings of the frontal, maxillary, and sphenoid sinuses to help restore drainage in a minimally invasive way. In addition Medtronic is planning clinical trials to expand the indications for use of the Nuvent product to additional procedures, such as revision surgery.
“Nuvent is very appealing to customers because of its novel built-in surgical EM navigation, ease of use, and the familiar sinus instrument styles,” said Vince Racano, vice president and general manager of the ENT division at Medtronic. “This system is another example of our innovative integration of technologies and therapies that brings ‘Medtronic Surgical Synergy’ to the operating room, driving procedural excellence, economic value, and patient care.”